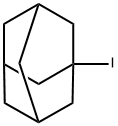
1-Iodoadamantane
- русский язык имя
- английское имя1-Iodoadamantane
- CAS №768-93-4
- CBNumberCB2191577
- ФормулаC10H15I
- мольный вес262.13
- номер MDLMFCD00134444
- файл Mol768-93-4.mol
химическое свойство
| Температура плавления | 75-76 °C(lit.) |
| Температура кипения | 265.7±9.0℃ (760 Torr) |
| плотность | 1.63±0.1 g/cm3 (20 ºC 760 Torr) |
| показатель преломления | 1.6610 (estimate) |
| Fp | 115.9±13.1℃ |
| температура хранения | 2-8°C(protect from light) |
| растворимость | DMSO: 52 mg/mL (198.37 mM);; |
| форма | solid |
| Стабильность | Stable, but light and moisture sensitive. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C10H15I/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6H2 |
| ИнЧИКей | PXVOATXCSSPUEM-UHFFFAOYSA-N |
| SMILES | C12(I)CC3CC(CC(C3)C1)C2 |
| FDA UNII | XT3Z54Z28A |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
1-Iodoadamantane химические свойства, назначение, производство
Химические свойства
solidИспользование
1-Iodoadamantane is suitable reagent used to evaluate the rate constants for the reduction of haloadamantanes by SmI2 in presence of hexamethylphosphoramide (HMPA) and H2O by GC/MS-analyzed method. It may be used as iodine atom donor for probing the intermediacy of radical to investigate the chemistry of highly reactive, strained systems such as propellane. It may be used as starting reagent in the synthesis of N-(1-adamantyl)acetamide via nucleophilic substitution. It may be employed in the free-radical carbonylation reactions with alkenes.Общее описание
1-Iodoadamantane is a haloadamantane. Voltammetric reduction of 1-iodoadamantane at a silver cathode in tetrahydrofuran (THF) and acetonitrile (ACN) is reported to involve a single electron forming a mixture of monomeric and dimeric products. The photoinduced reaction of 1-iodoadamantane in DMSO is reported to afford substitution products on C3, C6, and C8, 1-adamantanol, 1-adamantyl 2-naphthyl ether, and adamantine. The photostimulated reaction of the phthalimide anion with 1-iodoadamantane is reported to yield 3-(1-adamantyl) phthalimide and 4-(1-adamantyl) phthalimide, along with the reduction product adamantane. 1-Iodoadamantane is reported to undergoe photostimulated reaction with the enolate anion of acetone, acetophenone and propiophenone to give admantane and the substitution products.Методы очистки
Dissolve the iodide in Et2O, shake with aqueous NaHSO3, aqueous K2CO3, and H2O, dry (Na2SO4), evaporate and recrystallise it from MeOH at -70o (to avoid alcoholysis) to give white crystals. [Schleyer & Nicholas J Am Chem Soc 83 2700 1961, lit m of 151-152.5o is incorrect.] Also purify by recrystallisation from pet ether (40-60oC) followed by rigorous drying and repeated sublimation. [Beilstein 5 IV 470.]1-Iodoadamantane запасные части и сырье
1-Iodoadamantane поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-371-66670886 | China | 19902 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +8618530059196 | China | 11805 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +8615604608665 15604608665 |
CHINA | 9414 | 58 | |
| +86-592-5360779 +86-13055435203 |
China | 5988 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 |