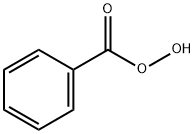
Пербензойная кислота
- английское имяPerbenzoic acid
- CAS №93-59-4
- CBNumberCB1852437
- ФормулаC7H6O3
- мольный вес138.12
- EINECS202-260-2
- номер MDLMFCD00216871
- файл Mol93-59-4.mol
химическое свойство
| Температура плавления | 41-43° |
| Температура кипения | bp15 100-110° (partial decomposition) |
| плотность | 1.2667 (rough estimate) |
| показатель преломления | 1.4600 (estimate) |
| пка | 7.98±0.40(Predicted) |
| цвет | Leaflets or platelets from pet ether |
| Справочник по базе данных CAS | 93-59-4 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | C0EZ97V99I |
Пербензойная кислота химические свойства, назначение, производство
Химические свойства
Volatile solid with a pungent odor; sublimes;mp 41–43°C (105.8–109°F); bp 100–105°C(212–221°F) at 15 torr (partially decomposes); slightly soluble in water but mixesreadily with most organic solvents.Использование
Perbenzoic acid is used to convert ethylenic compounds into oxides; in analysis of unsatd compounds, to determine the number of double bonds.Угроза здоровью
The toxicity of peroxybenzoic acid is verylow on animals. In humans it is almostnontoxic. Peroxybenzoic acid caused skintumor in mice on prolonged contact (NIOSH1986). Its tumorigenic action was lower thanthat of peroxyacetic acid. Its carcinogenicityin humans is unknown.Пожароопасность
The presence of the peroxide functional group renders its strong oxidizing properties similar to those of other peroxy compounds. However, this compound is not hazardous like peroxyacetic or peroxyformic acids. It is not shock sensitive. Violent decomposition may occur only at high temperatures and/or in conjunction with certain organic contaminants that are easily oxidizable. Although there is no report of its explosion, general safety measures for handling peroxy compounds should be followed.Профиль безопасности
Moderately irritating to skin, eyes, and mucous membranes by ingestion and inhalation. Questionable carcinogen with experimental tumorigenic data by skin contact. A dangerous fire hazard when exposed to heat, flame, or reducing materials. A powerful oxidМетоды очистки
Crystallise the peracid from *benzene or pet ether. It sublimes readily and is steam volatile. It is soluble in CHCl3, CCl4 and Et2O. [Braun Org Synth Coll Vol I 431 1941.] EXPLOSIVE.Пербензойная кислота запасные части и сырье
Пербензойная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 4009903999 13355009207 |
China | 18736 | 57 | |
| 0755-66853366; 13670046396 |
China | 24342 | 58 |