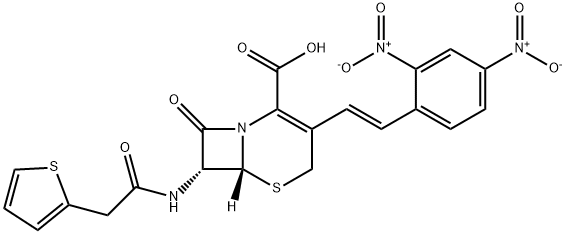
НИТРОЦЕФИН
- английское имяNitrocefin
- CAS №41906-86-9
- CBNumberCB1322600
- ФормулаC21H16N4O8S2
- мольный вес516.5
- номер MDLMFCD12165893
- файл Mol41906-86-9.mol
химическое свойство
| Температура плавления | 103-113° (dec); mp 167-169° (dec) (Lee) |
| альфа | D20 -224° (c = 1.0 in dioxane) |
| Температура кипения | 872.0±65.0 °C(Predicted) |
| плотность | 1.67±0.1 g/cm3(Predicted) |
| температура хранения | 2-8°C |
| растворимость | DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS (pH 7.2) (1:20): 0.04 mg/ml |
| форма | A crystalline solid |
| пка | 2.50±0.50(Predicted) |
| цвет | Yellow to orange |
| Стабильность | Hygroscopic, Unstable in Solution |
| ИнЧИКей | LHNIIDJCEODSHA-OQRUQETBSA-N |
| SMILES | N12[C@@]([H])([C@H](NC(CC3SC=CC=3)=O)C1=O)SCC(/C=C/C1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O)=C2C(O)=O |
| FDA UNII | EWP54G0J8F |
| UNSPSC Code | 12352200 |
| NACRES | NA.21 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
НИТРОЦЕФИН химические свойства, назначение, производство
Описание
The generation of β-Химические свойства
Nitrocefin is the chromogenic cephalosporin that acts as an excellent β-lactamase substrate. It exhibits a rapid distinctive color change from yellow (max at pH 7.0 = 390 nm) to red (max at pH 7.0 = 486 nm) as the amide bond in the beta-lactam ring is hydrolyzed by a β-lactamase. It is sensitive to hydrolysis by all known lactamases produced by Gram-positive and Gram-negative bacteria.Nitrocefin (Yellow) --β-lactamase-->Product (Red) (OD486nm)
Solution preparation and color change before and after β-lactamase exposure

(A) Concentrated nitrocefin (10.0 mg/mL) in DMSO before dilution with PBS buffer. (B) Nitrocefin diluted with PBS buffer to working concentration (1.0 mg/mL). The yellow color is indicative of intact, undegraded nitrocefin. (C) 25 units of betalactamase dropped on top of nitrocefin (1.0 mg/mL in PBS). The red color is the result of beta-lactamase mediated cleavage of the nitrocefin. (D) Vortexed mixture of contents shown in picture (C).
Использование
Nitrocefin is a chromogenic β-lactamase substrate that undergoes colour change from yellow to red as the amide bond in the β-Lactam ring is hydrolyzed by β-lactamase. Nitrocefin undergoes colour chang es induced by lactamases produced by Gram-positive and Gram-negative bacteria. Several studies have utilized the colour changing properties of Nitrocefin for the detection of β-lactamase activity from bacterial cell extracts by isoelectric focusing and spectroscopy. Nitrocefin has also been used in studies involving β-lactamase resistant antibiotics.Подготовка
Nitrocefin is a key reagent for high and low throughput assays of the activities of penicillin-binding proteins (PBPs) and β-lactamases, the former used for discovery of antibiotics and the latter for inhibitors of resistance determinants for β-lactam antibiotics. This compound is commercially available but is prohibitively expensive because of the circuitous routes to its synthesis. We describe herein a three-step synthesis of nitrocefin that gives an overall yield of 44%. This is a practical route to the synthesis of this key reagent for drug discovery.A Practical Synthesis of Nitrocefin
Биологические функции
In determination of b-lactamase activity in biological samples.Nitrocefin is a colorless or faint yellow cephalosporin antimicrobial that is hydrolyzed rapidly by most beta-lactamases.The hydrolysis product is pink.The bacterium to be tested is applied to a paper disk containing nitrocefin. A pink color developing within minutes (positive test) indicates a beta-lactamaseproducing bacterium.
НИТРОЦЕФИН запасные части и сырье
сырьё
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-(hydroxymethyl)-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-, diphenylmethyl ester, (6R,7R)-
- (6R,7R)-3-(Chloromethyl)-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid (4-Methoxyphenyl)methyl Ester
- Hydroxymethyl-7-Aminocephalosporanic acid
- 7-аминоцефалоспориновая кислота
- 2-Thiopheneacetyl хлорид
НИТРОЦЕФИН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8615116235351 | China | 16 | 58 | ||
| +86-13487087296 +86-13787794286 |
China | 37 | 58 | ||
| +86-0571-85134551 | China | 15352 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| 86-13657291602 | CHINA | 22963 | 58 | ||
| 18503026267 | CHINA | 9636 | 58 | ||
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | ||
| +8613367258412 | China | 10319 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | ||
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |