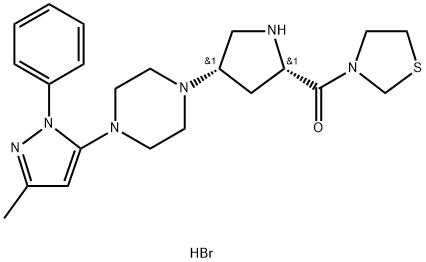
Teneligliptin Hydrobromide
- русский язык имя
- английское имяTeneligliptin Hydrobromide
- CAS №906093-29-6
- CBNumberCB12666692
- ФормулаC22H31BrN6OS
- мольный вес507.5
- EINECS620-495-1
- файл Mol906093-29-6.mol
химическое свойство
| Температура плавления | >211°C (dec.) |
| температура хранения | Refrigerator |
| растворимость | Methanol (Slightly) |
| форма | Solid |
| цвет | White to Light Beige |
| оптическая активность | [α]/D -28 to -36°, c =0.5 in methanol |
| Растворимость в воде | H2O: 2mg/mL, clear |
| FDA UNII | 556RZT8JPF |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H413:Может вызвать долгосрочные отрицательные последствия для водных организмов.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Teneligliptin Hydrobromide химические свойства, назначение, производство
Использование
Teneligliptin Hydrobromide (2:5) is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is used to treat type 2 diabetes. It is eliminated via excretion, and has a half-life of 24.2 hours in the human body.Клиническое использование
Teneligliptin is a DPP-4 inhibitor which was approved in Japan in 2012 for the treatment of type II diabetes. It was discovered and developed by Mitsubishi Tanabe Pharma under the trade name Tenelia®. Similar to other marketed DPP-4 inhibitors, teneligliptin was well tolerated in all studies and QD dosing produced a long-lasting inhibitory action against DPP-4 and an increase in active GLP-1 levels, with very low rates of renal excretion.Синтез
The only reported synthesis of teneligliptin is described in the scheme below. Reaction of commercially available N-Boc-piperazine (158) with diketene (159) in DMF at room temperature gave acetoacetamide 160 in 86% yield, and this material was immediately condensed with phenylhydrazine in methanesulfonic acid followed by a cyclodehydration with phosphorus oxychloride to give pyrazole 161 in 12% yield. The t-butyl carbamate was then removed with TFA in dichloromethane to give amine 162 in 88% yield. This amine was then subjected to butyrolactam 165 (which was prepared from N-Boctrans- 4-hydroxy-L-proline (163) coupled with thiazolidine (164) under conventional amide-forming conditions using EDC) in the presence of sodium triacetoxy borohydride (STAB-H) in acetic acid. This reductive amination reaction afforded the cis-aminopyrrolidine 166 exclusively in 50% yield. Removal of the t-butyl carbamate group with TFA afforded the teneligliptin free amine in 93% yield, and this freebase was then subsequently treated with 48% hydrobromic acid in refluxing ethanol to give teleligliptin hydrobromide hydrate (XXV) in 90% yield.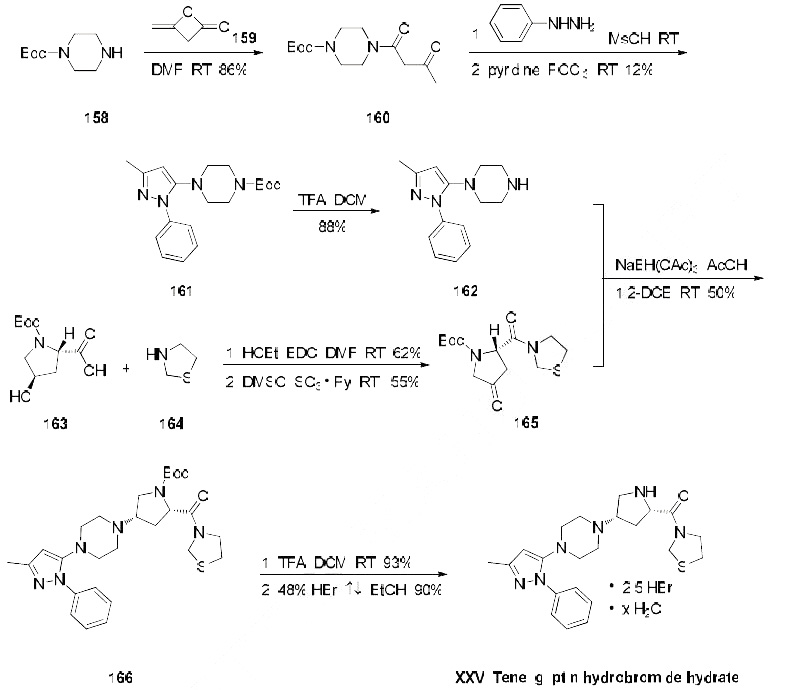
Teneligliptin Hydrobromide поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | |
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | |
| 010-60279497 | CHINA | 1803 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-21-33585366 - 03@ | CHINA | 738 | 60 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| 0086-182-6772-3597 | CHINA | 419 | 58 |