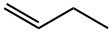
1-бутен
- английское имя1-BUTENE
- CAS №106-98-9
- CBNumberCB0305491
- ФормулаC4H8
- мольный вес56.11
- EINECS203-449-2
- номер MDLMFCD00009383
- файл Mol106-98-9.mol
химическое свойство
| Температура плавления | −185 °C(lit.) |
| Температура кипения | −6.3 °C(lit.) |
| плотность | 0.5951 |
| плотность пара | 1.93 (vs air) |
| давление пара | 1939 mm Hg ( 21.1 °C) |
| показатель преломления | 1.3962 |
| Fp | 80 |
| температура хранения | -20°C Freezer |
| растворимость | Soluble in alcohol, benzene, and ether (Weast, 1986) |
| пка | >14 (Schwarzenbach et al., 1993) |
| форма | gas |
| цвет | Colorless to Almost colorless |
| Запах | Slightly aromatic |
| Порог?обнаружения?запаха? | 0.36ppm |
| Пределы взрываемости | 9.3% |
| Растворимость в воде | 222 mg/kg at 25 °C (shake flask-GC, McAuliffe, 1966) |
| Точка замерзания | -185.35℃ |
| Мерк | 14,1519 |
| БРН | 1098262 |
| констант закона Генри | (atm?m3/mol): 0.25 at 25 °C (Hine and Mookerjee, 1975) |
| Стабильность | Volatile |
| ИнЧИКей | VXNZUUAINFGPBY-UHFFFAOYSA-N |
| LogP | 2.35 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | 1-BUTENE |
| Справочник по базе данных CAS | 106-98-9(CAS DataBase Reference) |
| FDA UNII | LY001N554L |
| Система регистрации веществ EPA | 1-Butene (106-98-9) |
больше
| Коды опасности | F+,F | |||||||||
| Заявления о рисках | 12 | |||||||||
| Заявления о безопасности | 9-16-33 | |||||||||
| РИДАДР | UN 1012 2.1 | |||||||||
| WGK Германия | - | |||||||||
| F | 4.5-31 | |||||||||
| Температура самовоспламенения | 723 °F | |||||||||
| Примечание об опасности | Extremely Flammable | |||||||||
| Классификация DOT | 2.1 (Flammable gas) | |||||||||
| Класс опасности | 2.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 2901230000 | |||||||||
| Банк данных об опасных веществах | 106-98-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H220:Чрезвычайно легковоспламеняющийся газ.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
1-бутен химические свойства, назначение, производство
Описание
1-Butene is a colourless, stable but polymerises exothermically, extremely flammable liquefied gas with an aromatic odour. It is insoluble in water and is one of the isomers of butane. 1-Butene readily forms explosive mixtures with air. It is incompatible with strong oxidising agents, halogens, halogen acids, metal salts, boron trifluoride, fluorine, and nitrogen oxides. 1-Butene of high purity is made by cracking naphtha and separating it from other products by an extra-high-purity distillation column. It is an important organic compound in the production of several industrial materials – for instance, linear low-density polyethylene (LLDPE), a more flexible and resilient polyethylene, and a range of polypropylene resins – and in the production of polybutene, butylene oxide, and the C4 solvents secondary butyl alcohol (SBA) and MEK. The vapour of 1-butene is heavier than air and may travel long distances to an ignition source and flash back.Химические свойства
1-Butene is a colorless, extremely flammable liquefi ed gas with an aromatic odor. It is insol uble in water and is an isomer of butane. It is highly flammable and readily forms explo sive mixtures with air. 1-Butene of high purity is made by cracking naphtha and separating it from other products by an extra-high purity distillation column. However, 1-butene is incompatible with metal salts, fl uorine, nitrogen oxides, boron trifl uoride, halogen acids, halogens, and strong oxidizing agents. It is an important organic compound in the produc tion of several industrial materials, i.e., linear low density polyethylene (LLDPE), a more fl exible and resilient polyethylene, a range of polypropylene resins, and in the production of polybutene, butylene oxide and in the C4 solvents, secondary butyl alcohol (SBA) and methyl ethyl ketone (MEK). The vapor of 1-butene is heavier than air and may travel long distances to an ignition source and fl ash back.Физические свойства
Butenes or butylenes are hydrocarbon alkenes that exist as four different isomers. Each isomer is a flammable gas at normal room temperature and one atmosphere pressure, but their boiling points indicate that butenes can be condensed at low ambient temperatures and/or increase pressure similar to propane and butane. The “2” designation in the names indicates the position of the double bond. The cis and trans labels indicate geometric isomerism. Geometric isomers are molecules that have similar atoms and bonds but different spatial arrangement of atoms. The structures indicate that three of the butenes are normal butenes, n-butenes, but that methylpropene is branched. Methylpropene is also called isobutene or isobutylene. Isobutenes are more reactive than n-butenes, and reaction mechanisms involving isobutenes differ from those of normal butenes.Использование
Butenes are used extensively in gasoline production to produce high-octane gasoline compounds.Another large use of normal butenes in the petrochemical industry is in the production of 1,3-butadiene. Butene is used in the plastics industry to make both homopolymers and copolymers. Another use of 1-butene is in the production of solvents containing four carbons such as secondary butyl alcohol and methyl ethyl ketone (MEK).Методы производства
Most butenes are produced in the cracking process in refineries along with other C-4 fractions such as the butanes. Butenes are separated from other compounds and each other by several methods. Isobutene is separated from normal butanes by absorption in a sulfuric acid solution. Normal butenes can be separated from butanes by fractionation. The close boiling points of butanes and butenes make straight fractional distillation an inadequate separation method, but extractive distillation can be used. Butenes can also be prepared from the dehydrogenation (elimination of hydrogen) of butane.Определение
ChEBI: A butene with unsaturation at position 1.Общее описание
Colorless gas.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
The unsaturated aliphatic hydrocarbons, such as 1-BUTENE, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions. May react with oxidizing materials. Aluminum borohydride reacts with alkenes and in the presence of oxygen, combustion is initiated even in the absence of moisture.Угроза здоровью
Exposures to 1-butene cause the effects of an asphyxiant and/or an anesthetic (at high concentrations). Workers exposed to 1-butene develop eye irritation.Пожароопасность
1-BUTENE is flammable. Vapor is heavier than air and may travel long distances to an ignition source and flash back.Экологическая судьба
Biological. Biooxidation of 1-butene may occur yielding 3-buten-1-ol, which may oxidize to give 3-butenoic acid (Dugan, 1972). Washed cell suspensions of bacteria belonging to the genera Mycobacterium, Nocardia, Xanthobacter, and Pseudomonas and growing on selected alkenes metabolized 1-butene to 1,2-epoxybutane (Van Ginkel et al., 1987).Photolytic. Products identified from the photoirradiation of 1-butene with nitrogen dioxide in air are epoxybutane, 2-butanone, propanal, ethanol, ethyl nitrate, carbon monoxide, carbon dioxide, methanol, and nitric acid (Takeuchi et al., 1983).
The following rate constants were reported for the reaction of 1-butene and OH radicals in the atmosphere: 1.0 x 10-17 cm3/molecule?sec (Bufalini and Altshuller, 1965); 2.70 x 10-11 cm3/molecule?sec (Atkinson et al., 1979); 3.14 x 10-11 cm3/molecule?sec (Atkinson, 1990; Sablji? and Güsten, 1990). Reported photooxidation reaction rate constants for the reaction of 1-butene and ozone are 1.23 x 10-17, 1.0 x 10-17, 1.03 x 10-17 cm3/molecule?sec (Adeniji et al., 1981). Based on the reaction of 1-butene and OH radicals gas phase, the atmospheric lifetime was estimated to be 5.5 h in summer sunlight.
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Incomplete combustion will generate carbon monoxide.
Hydrolysis in water is not expected to be signicant because 1-butene is very volatile.
Меры предосторожности
When working with 1-butene, occupational workers should wear proper protectives, preferably a NIOSH-approved full-face positive pressure supplied-air respirator or a self contained breathing apparatus (SCBA). Workers should not wear contact lenses.1-бутен запасные части и сырье
сырьё
1of4
запасной предмет
1-бутен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +8613817748580 | China | 40066 | 58 | |
| +undefined+86-19307248857 | China | 982 | 58 | |
| +8618950047208 | China | 43416 | 58 |
1-бутен Обзор)
- Платина(II) ацетилацетона
- Алюминия ацетилацетона
- Трис (2,2,6,6-тетраметил-3 ,5-heptanedionato) европий (III)
- Ванадил(IV) ацетилацетонат
- Трис (2,2,6,6-тетраметил-3 ,5-heptanedionato) диспрозий (III)
1of4