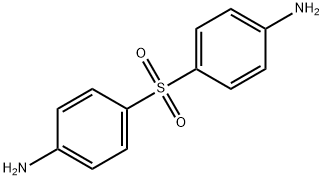
4,4 '-диаминодифенилсульфон
- английское имя4,4'-Diaminodiphenylsulfone
- CAS №80-08-0
- CBNumberCB0152851
- ФормулаC12H12N2O2S
- мольный вес248.3
- EINECS201-248-4
- номер MDLMFCD00007887
- файл Mol80-08-0.mol
| Температура плавления | 175-177 °C(lit.) |
| Температура кипения | 511.7±35.0 °C(Predicted) |
| плотность | 1.2701 (rough estimate) |
| Плотность накопления | 250-350kg/m3 |
| давление пара | 0.004Pa at 25℃ |
| показатель преломления | 1.5950 (estimate) |
| температура хранения | 2-8°C |
| растворимость | 0.38g/l |
| форма | Crystalline Powder |
| пка | pKb 13.0(at 25℃) |
| цвет | White to beige |
| РН | 5.5-7.5 (H2O, 20℃)(saturated aqueous solution) |
| Растворимость в воде | <0.1 g/100 mL at 20 ºC |
| Мерк | 14,2822 |
| БРН | 788055 |
| BCS Class | 4,2 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | MQJKPEGWNLWLTK-UHFFFAOYSA-N |
| LogP | 0.97 at 25℃ |
| Справочник по базе данных CAS | 80-08-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | dapsone; DDS |
| FDA UNII | 8W5C518302 |
| Словарь наркотиков NCI | Avlosulfon |
| Код УВД | D10AX05,J04BA02 |
| Справочник по химии NIST | Dapsone(80-08-0) |
| МАИР | 3 (Vol. 24, Sup 7) 1987 |
| Система регистрации веществ EPA | Dapsone (80-08-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22 | |||||||||
| Заявления о безопасности | 22 | |||||||||
| РИДАДР | 3249 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | BY8925000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29051620 | |||||||||
| кода HS | 29309070 | |||||||||
| Банк данных об опасных веществах | 80-08-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1000 mg/kg LD50 dermal Rabbit > 4000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H411:Токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H370:Поражает органы (Глаза) в результате однократного воздействия.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P311:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
4,4 '-диаминодифенилсульфон химические свойства, назначение, производство
Химические свойства
Off -White Crystalline SolidИспользование
4,4'-diaminodiphenylsulfone be used for preparation polyimide and epoxy resin material.Показания
Although dapsone (Avlosulfon) is most often used as an antimicrobial agent, it has important antiinflammatory properties in many noninfectious skin diseases. The mechanism of action of dapsone in skin disease is not clear.Most of the cutaneous diseases for which it is effective manifest inflammation and are characterized by an infiltration of neutrophils; the drug’s antiinflammatory effect may arise from its inhibition of intracellular neutrophil reactions mediated by myeloperoxidase and hydrogen peroxide or from its scavenging of reactive oxygen species, which inhibits inflammation.Определение
ChEBI: A sulfone that is diphenylsulfone in which the hydrogen atom at the 4 position of each of the phenyl groups is substituted by an amino group. It is active against a wide range of bacteria, but is mainly employed for its actions against Mycobacteriu leprae, being used as part of multidrug regimens in the treatment of all forms of leprosy.Антимикробная активность
Dapsone is active against many bacteria and some protozoa. Fully susceptible strains of M. leprae are inhibited by a little as 0.003 mg/L. It is predominantly bacteristatic. Resistance is associated with mutations in the folP1 gene involved in the synthesis of para-aminobenzoic acid.Приобретенная устойчивость
Resistance to high levels is acquired by several sequential mutations. As a result of prolonged use of dapsone monotherapy, acquired resistance emerged in patients with multibacillary leprosy in many countries. Initial resistance also occurs in patients with both paucibacillary and multibacillary leprosy. Thus, leprosy should always be treated with multidrug regimens. Resistance of M. leprae to dapsone (and other anti-leprosy drugs) may now be determined by use of DNA microarrays.Общее описание
Odorless white or creamy white crystalline powder. Slightly bitter taste.Реакции воздуха и воды
Sensitive to oxidation and light. Insoluble in water.Профиль реактивности
4,4'-Diaminodiphenylsulfone can neutralize acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated in combination with strong reducing agents, such as hydrides. Incompatible with strong oxidizing agents. Also incompatible with epoxy resins under uncontrolled conditions .Пожароопасность
4,4'-Diaminodiphenylsulfone is probably combustible.Фармацевтические приложения
The most effective of a number of sulfonamide derivatives to be tested against leprosy. The dry powder is very stable. It is only slightly soluble in water.Фармакокине?тика
Oral absorption: >90%Cmax 100 mg oral: c. 2 mg/L after 3–6 h
Plasma half-life: 10–50 h
Plasma protein binding: c. 50%
It is slowly but almost completely absorbed from the intestine and widely distributed in the tissues, but selectively retained in skin, muscle, kidneys and liver. It is metabolized by N-oxidation and also by acetylation, which is subject to the same genetic polymorphism as isoniazid. The elimination half-life is consequently very variable, but on standard therapy the trough levels are always well in excess of inhibitory concentrations. It is mostly excreted in the urine: in the unchanged form (20%), as N-oxidation products (30%) and as a range of other metabolites.
Клиническое использование
Leprosy (multidrug regimens)Prophylaxis of malaria, treatment of chloroquine-resistant malaria (in combination with pyrimethamine)
Prophylaxis of toxoplasmosis (in combination with pyrimethamine)
Prophylaxis (monotherapy) and treatment (in combination with trimethoprim) of Pneumocystis jirovecii pneumonia
Dermatitis herpetiformis and related skin disorders
Побочные эффекты
Although usually well tolerated at standard doses, gastrointestinal upsets, anorexia, headaches, dizziness and insomnia may occur. Less frequent reactions include skin rashes, exfoliative dermatitis, photosensitivity, peripheral neuropathy (usually in non-leprosy patients), tinnitus, blurred vision, psychoses, hepatitis, nephrotic syndrome, systemic lupus erythematosus and generalized lymphadenopathy.The term ‘dapsone syndrome’ is applied to a skin rash and fever occurring 2–8 weeks after starting therapy and sometimes accompanied by lymphadenopathy, hepatomegaly, jaundice and/or mononucleosis.
Blood disorders include anemia, methemoglobinemia, sulfhemoglobinemia, hemolysis (notably in patients with glucose- 6-phosphate dehydrogenase deficiency), mononucleosis, leukopenia and, rarely, agranulocytosis. Severe anemia should be treated before patients receive dapsone.
The incidence of adverse reactions declined in the 1960s but reappeared around 1982 when multidrug therapy was introduced, and may represent an unexplained interaction with rifampicin.
Профиль безопасности
Poison by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: agranulocytosis, change in tubules and other kidney changes, cyanosis, effect on joints, hemolysis with or without anemia, jaundice, methemoglobinemiacarboxyhemoglobinemia, retinal changes, somnolence. Experimental reproductive effects. Can cause hepatitis, dermatitis, and neuritis. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Human mutation data reported. Used in leprosy treatment and veterinary medicine. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also SULFONATES.4,4 '-диаминодифенилсульфон запасные части и сырье
4,4 '-диаминодифенилсульфон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 28-85356300 +8618980937689 |
CHINA | 94 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-18186686046 +86-18186686046 |
China | 5861 | 58 |