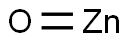Zinc oxide: Properties, application and safety

Figure 1 Appearance of Zinc oxide.
Application
The applications of zinc oxide powder are numerous. Most applications exploit the reactivity of the oxide as a precursor to other zinc compounds. For material science applications, zinc oxide has high refractive index, high thermal conductivity, binding, antibacterial and UV-protection properties. Consequently, it is added into materials and products including plastics, ceramics, glass, cement, rubber, lubricants, paints, ointments, adhesive, sealants, concrete manufacturing, pigments, foods, batteries, ferrites, fire retardants, etc [6]. Between 50% and 60% of ZnO use is in the rubber industry. Zinc oxide along with stearic acid is used in the vulcanization of rubber ZnO additive also protect rubber from fungi (see medical applications) and UV light. In addition, Zinc oxide as a mixture with about 0.5% iron(III) oxide (Fe2O3) is called calamine and is used in calamine lotion. Two minerals, zincite and hemimorphite, have been historically called calamine. When mixed with eugenol, a ligand, zinc oxide eugenol is formed, which has applications as a restorative and prosthodontic in dentistry [7].
Safety
As a food additive, zinc oxide is on the U.S. FDA's list of generally recognized as safe, or GRAS, substances. Zinc oxide itself is non-toxic; it is hazardous, however, to inhale zinc oxide fumes, such as generated when zinc or zinc alloys are melted and oxidized at high temperature. This problem occurs while melting alloys containing brass because the melting point of brass is close to the boiling point of zinc. Exposure to zinc oxide in the air, which also occurs while welding galvanized (zinc plated) steel, can result in a malady called metal fume fever. For this reason, typically galvanized steel is not welded, or the zinc is removed first [8].
[2]Bacaksiz, E.; Parlak, M.; Tomakin, M.; ?zcelik, A.; Karakiz, M.; Altunbas, M. The effect of zinc nitrate, zinc acetate and zinc chloride precursors on investigation of structural and optical properties of ZnO thin films. J. Alloy. Compd 2008, 466, 447–450.
[3]Wang, Z.L. Splendid one-dimensional nanostructures of zinc oxide: A new nanomaterial family for nanotechnology. ACS Nano 2008, 2, 1987–1992.
[4]Phillips JC. Ionicity of the Chemical Bond in Crystals". Reviews of Modern Physics. 1970, 42 (3): 317–356.
[5]Klingshirn C. ZnO: material, physics and applications. ChemPhysChem. 2007, 8 (6): 782–803.
[6]Ambica Dhatu Private Limited. Applications of ZnO. Archived December 19, 2019, at the Wayback Machine Access date January 25, 2009.
[7]Nicholson JW. The chemistry of cements formed between zinc oxide and aqueous zinc chloride. Journal of Materials Science. 1998, 33 (9): 2251–2254.
[8]Zheng X, Shen G, Wang C, Li Y, Dunphy D, Hasan T, et al. Bio-inspired Murray materials for mass transfer and activity. Nature Communications. 2017, 8: 14921.
You may like
Related articles And Qustion
Lastest Price from Zinc oxide manufacturers

US $1200.00-1100.00/ton2025-09-18
- CAS:
- 1314-13-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00-0.00/g2025-06-11
- CAS:
- 1314-13-2
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 20000000t