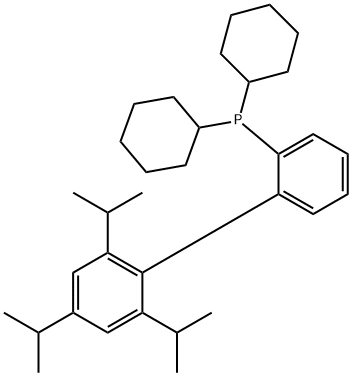
X-PHOS: Properties and Applications as Catalyst
Description
X-PHOS is a phosphine ligand derived from biphenyl. Its palladium complexes exhibit high activity for Buchwald-Hartwig amination reactions involving aryl chlorides and aryl tosylates.

Properties
X-PHOS is characterized by its remarkable stability and reactivity, emerges as a crucial ligand in catalytic applications, particularly in palladium-catalyzed borylation reactions.
Applications as Catalyst
as a Ligand used in a Pd-catalyzed Suzuki coupling leading to C-15 analogs of vindoline.1
Direct annulation of 2-haloanilines to indoles and tryptophans catalyzed by Pd. Synthesis of regioregular polythiophenes.
Preferred ligand for greener Sonogashira coupling in TPGS-750-M.
Application of X-PHOS in Palladium-Catalyzed Borylation Reactions
X-PHOS, a phosphine ligand, has shown significant promise in enhancing the efficiency of palladium-catalyzed Miyaura borylation reactions, a critical method for producing aryl boronic acids and esters, essential intermediates in the synthesis of active pharmaceutical ingredients (APIs). This paper demonstrates how using X-PHOS in such reactions enables significant improvements under milder conditions and with lower catalyst loadings. Typically, palladium catalysts require precise ligand control to achieve high activity and selectivity; X-PHOS offers this by providing a robust framework that stabilizes the palladium center, thus facilitating the activation of aryl halides. The advantage of using X-PHOS in the borylation process is evident in the ability to operate at lower temperatures (around 35°C) and complete reactions in less than two hours with a minimal palladium loading of 0.5 mol%. These improvements are not only cost-effective but also reduce the environmental impact associated with metal catalysts. 2, 3
References:
[1] PETER D. JOHNSON V H R Jeong Hun Sohn. Synthesis of C-15 Vindoline Analogues by Palladium-Catalyzed Cross-Coupling Reactions[J]. Journal of Organic Chemistry, 2006, 71 20: 7499-7930. DOI:10.1021/jo061243y.[2] SANTIAGO BARROSO. Improvement in the Palladium-Catalyzed Miyaura Borylation Reaction by Optimization of the Base: Scope and Mechanistic Study[J]. Journal of Organic Chemistry, 2020, 86 1: 1-1310. DOI:10.1021/acs.joc.0c01758.
[3] IRINI ABDIAJ*, JESUS ALCáZAR*. End-to-End Automated Synthesis of C(sp3)-Enriched Drug-like Molecules via Negishi Coupling and Novel, Automated Liquid–Liquid Extraction[J]. Journal of Medicinal Chemistry, 2022, 66 1: 1-1082. DOI:10.1021/acs.jmedchem.2c01646.
You may like
Related articles And Qustion
See also
Lastest Price from X-PHOS manufacturers

US $10.00/KG2025-04-21
- CAS:
- 564483-18-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons
US $0.00-0.00/KG2025-04-21
- CAS:
- 564483-18-7
- Min. Order:
- 5KG
- Purity:
- 98%
- Supply Ability:
- 25KG