Why thiophene is commonly used in drugs?
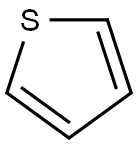
Thiophene is a five-membered heteroaromatic compound containing a sulfur atom at 1 position. It is considered to be a structural alert with formula C4H4S, chemical name is thiacyclopentadiene.
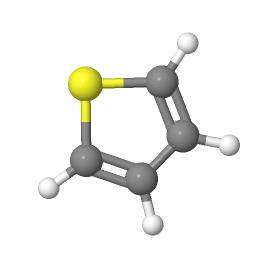
Thiophene was discovered as a contaminant in benzene. It has a molecular mass of 84.14 g/mol, density is 1.051 g/ml and melting point is − 38°C. It is soluble in most organic solvents like alcohol and ether but insoluble in water. The “electron pairs” on sulfur are significantly delocalized in the π electron system and behave highly reactive like benzene derivative. Thiophene forms an azeotrope with ethanol like benzene. The similarity between the physicochemical properties of benzene and thiophene is remarkable. For example, the boiling point of benzene is 81.1°C and that of thiophene is 84.4°C (at 760 mmHg) and therefore, both are well known examples of bioisosterism. It can be easily sulfonated, nitrated, halogenated, acylated but cannot be alkylated and oxidized.
In medicinal chemistry, thiophene derivatives are extremely significant heterocycles exhibiting remarkable applications in different disciplines. In medicine, thiophene derivatives show antimicrobial, analgesic and anti-inflammatory, antihypertensive, and antitumor activity while they are also used as inhibitors of corrosion of metals or in the fabrication of light-emitting diodes in material science.
You may like
Related articles And Qustion
Lastest Price from Thiophene manufacturers

US $20.00/kg2025-04-21
- CAS:
- 110-02-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/KG2025-01-03
- CAS:
- 110-02-1
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 2000tons