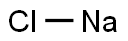ChemicalBook > Articles Catagory List >Inorganic-salts >why-does-sodium-chloride-produce-a-yellow-flame
Why does sodium chloride produce a yellow flame?
Jan 25,2024
Sodium flame test. Positive result of a flame test for sodium (Na), producing a yellow colour. This is due to the excitation of electrons in the sodium by the heat of the flame. As these electrons lose their energy, they emit photons of yellow light. The colour of the flame is different for different elements and can be used to identify unknown substances. Halides are typically more volatile than other salts, making them good for this test. Sodium chloride (NaCl) gives a bright yellow-orange in a flame test.

In the hot flame, some of the sodium ions regain their electrons to form neutral sodium atoms again. A sodium atom in an unexcited state has the structure 1s22s22p63s1, but within the flame there will be all sorts of excited states of the electrons.
Sodium's familiar bright orange-yellow flame colour results from promoted electrons falling back from the 3p1 level to their normal 3s1 level.
You may like
The Applications of Sodium tungstate dihydrate
Oct 10, 2025
Related articles And Qustion
See also
Lastest Price from Sodium chloride manufacturers
Sodium chloride

US $0.00-0.00/kg2025-12-13
- CAS:
- 7647-14-5
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 1000kg
Sodium chloride

US $10.00/KG2025-04-21
- CAS:
- 7647-14-5
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt