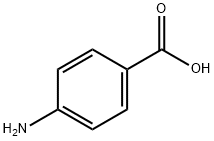
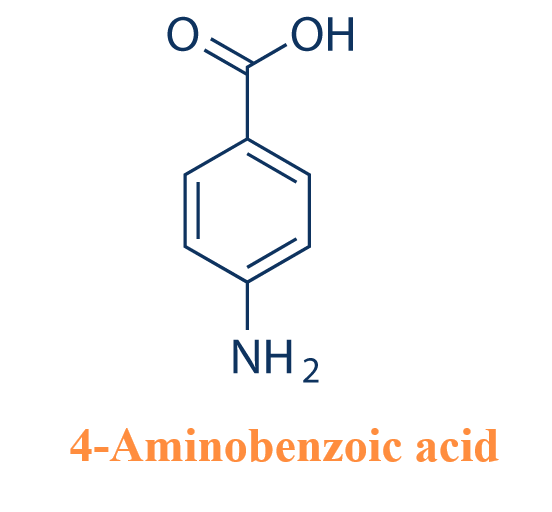
What is the pH and pKa of 4-Aminobenzoic acid?
4-Aminobenzoic acid, also known as p-aminobenzoic acid, abbreviated as PABA, is an acidic compound. PABA has a pH (0.5% solution) of 3.5; pKa = 4.87, and dissociates in two phases: pKa1 = 2.38 (at 25 °C) and pKa2 = 4.85 (at 25 °C).
4-Aminobenzoic acid (4-ABA) is used as an electrolyte additive to enhance the electrochemical performance of sulfated polyacrylonitrile cathodes in ether electrolytes. Compared with the blank electrolyte, the cell containing 4-ABA showed better cycling performance with a reversible capacity of 1178.73 mAh g-1 and capacity retention of 88.81% after 100 cycles at 0.5 C. 4-ABA can also be used for the effect of chemical modification on the adsorption characteristics of Cu(II) ions on cellulose. The modification was carried out by oxidising cellulose with sodium meta-iodate to form dialdehyde cellulose, which was then interacted with 4-aminobenzoic acid and converted into a derivative capable of chelating heavy metal ions.
References:
[1] XIAOJIA ZHENG. 4-Aminobenzoic acid as an electrolyte additive for enhancing the electrochemical properties of the sulfurized polyacrylonitrile cathode in ether electrolyte[J]. Ionics, 2023, 29 9. DOI:10.1007/s11581-023-05118-4.[2] T. E. NIKIFOROVA E. N K V A Kozlov. The Influence of Chemical Modification of Cellulose with 4-Aminobenzoic Acid on Sorption of Cu(II) Ions[J]. Protection of Metals and Physical Chemistry of Surfaces, 2021, 57 4. DOI:10.1134/S2070205121040195.
Related articles And Qustion
See also
Lastest Price from 4-Aminobenzoic acid manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 150-13-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $1.00-4.00/KG2025-09-11
- CAS:
- 150-13-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG