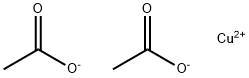
What is the formula for Copper(II) acetate?
Copper(II) acetate, with the molecular formula C4H6CuO4, generally exists in the form of monohydrate, with the appearance of blue-green powdery crystals, losing crystal water at 240°C, soluble in ethanol, slightly soluble in ether and glycerol, used as analytical reagents, chromatographic analysis reagents, and can also be used as organic synthesis catalysts, paint quick-drying agents, pesticide adjuvants, enamel pigment raw materials, etc.

What is Copper(II) acetate used for?
Copper(II) acetate has a variety of uses:
Organic reaction catalyst: used as a catalyst for various organic reactions, especially the synthesis of certain dyes and drugs.
Wood preservative: Historically, Copper(II) acetate has been used as a wood preservative to prevent wood from rotting and insect pests, making wood appear green, and is famous for protecting the hull.
Electroplating: used in electroplating processes to deposit a layer of copper on various substrates.
Click chemistry catalyst: Copper(II) acetate is used as a catalyst for click chemistry reactions, which involve the rapid, selective and high-yield formation of covalent bonds between structural units.
Chemical education: In educational environments, Copper(II) acetate is sometimes used in chemical demonstrations and experiments.
It is important to note that despite these uses, Copper(II) acetate should be handled with care, as copper compounds can be toxic. Precautions should be taken to avoid contact and contamination. Always follow safety guidelines and take appropriate protective measures when working with this compound.
Applications
Copper(II) acetate can act as an oxidant for carbanions, free radicals, and hydrocarbon compounds, enabling oxidative coupling reactions of electronegative substrates and solvent cleavage reactions of Si-C, Bi-C, Pb-C, and Sb-C bonds. It can also be used for the cyclopropanation of olefins with diazo esters or as a Lewis acid. Copper(II) acetate has oxidative activity toward carbanions. In the presence of pyridine, terminal alkynes can undergo oxidative coupling reactions to form diyne compounds.
You may like
See also
Lastest Price from Copper(II) acetate manufacturers

US $1200.00-1100.00/ton2025-08-11
- CAS:
- 142-71-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $6.00/kg2025-04-21
- CAS:
- 142-71-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month