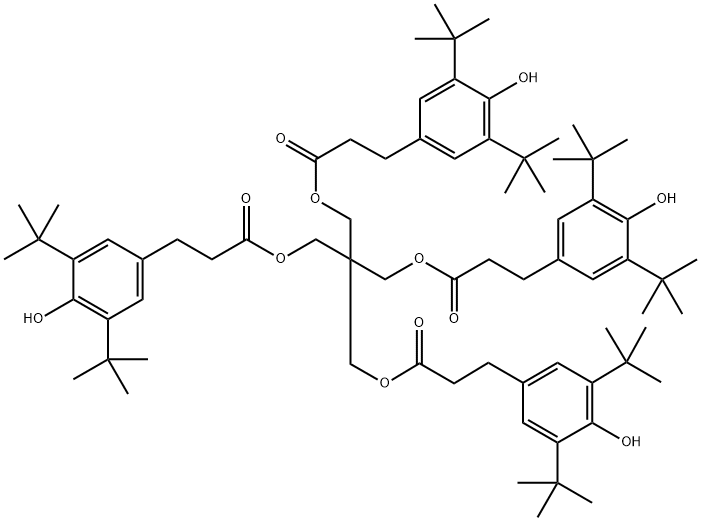
What is the degradation of Antioxidant 1010?
Plastic bonded explosive (PBX) 9501 formulation is comprised of, by weight, 94.9% cyclotetramethylene-tetranitramine (HMX), 2.5% poly(urethane ester) (Estane®5703) (called Estane hereinafter), 2.5% nitroplasticizer (NP), and ∼0.1 wt% Irganox 1010 (Irg1010; Antioxidant 1010) (used as an antioxidant)[1]. Previous studies have shown that Estane and other constituents of PBX 9501, such as NP and Irg1010, degrade over time, altering material properties. For Irganox 1010 (an ester-based antioxidant), hydrolysis was the preferred degradation mechanism, leading to products with increased solubility in water. Therefore, this stabilizer is less suitable for materials intended for water applications[2].
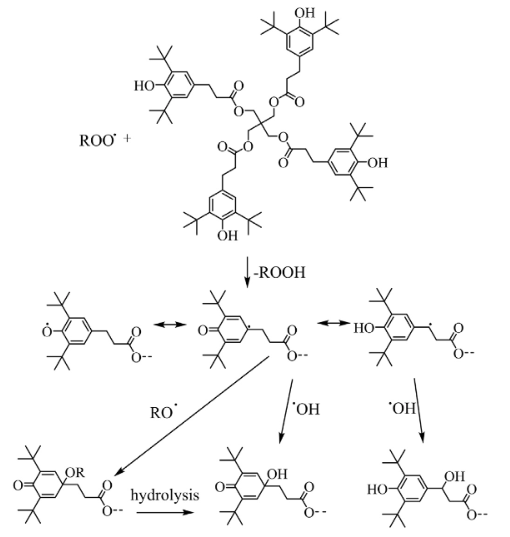
The degradation of Antioxidant 1010 has been widely studied in a series of comprehensive ageing studies of PBX 9501. Irganox 1010 has been shown to have minimal effect in protecting against moderate cross-linking but does prevent de-polymerization. Transformation to quinone structures played only a minor role in the case of Irganox 1010. Instead, hydrolysis of the ester bonds was the preferred degradation mechanism, which is an unwanted side reaction as it reduces the antioxidant efficiency without adding to the protection of the polymer, as already indicated above. This is aggravated by the fact that the formed products with free hydroxyl groups show an increased solubility in water. Even though subsequent transformation products still have antioxidant capacity, they are rendered useless as they are easily leached out from the polymeric material[3]. Nevertheless, it is known that sterically hindered phenols are not only effective hydrogen donors but may also undergo numerous further chemical reactions that inhibit the autoxidation of the polymer. In the aged polymer samples containing Irganox 1010, a previously unknown degradation mechanism was observed. Two peaks with similar MS spectra but higher molecular weight than the intact molecule were found. The mass differences of 16 and 32 respectively can be explained by additional oxygen chemically linked to the molecules. Following the above figure, it is assumed thatt the formation is due to the reaction of a phenoxyl radicalwith a a hydroxyl or alkoxy radical, which are formed in apreceding decompositionon of a hydroperoxide. The hydroperoxide is formed through hydrogen abstraction of a peroxy radical from the poly-olefin polymer backbone. These degradation products prove that Irganox 1010 not only acts as a hydrogen donor but also as a radical scavenger, thus further contributing to avoiding polymer degradation.
References:
[1] DALI YANG . The behavior of antioxidant irganox 1010 during the thermal degradation of a plastic bonded explosive[J]. Polymer Degradation and Stability, 2022, 200. DOI:10.1016/j.polymdegradstab.2022.109928.[2] FREYE C E. Decomposition of Irganox 1010 in plastic bonded explosives[J]. Journal of Applied Polymer Science, 2022, 139 30. DOI:10.1002/app.52686.
[3] SUSANNE BEISSMANN . Monitoring the degradation of stabilization systems in polypropylene during accelerated aging tests by liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry[J]. Polymer Degradation and Stability, 2013, 98 9: 1549-1952. DOI:10.1016/j.polymdegradstab.2013.06.015.
You may like
Related articles And Qustion
Lastest Price from Antioxidant 1010 manufacturers

US $0.00-0.00/KG2025-11-21
- CAS:
- 6683-19-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $3.00-9.00/KG2025-07-04
- CAS:
- 6683-19-8
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available