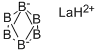What is the crystal structure of LaB6?
Lanthanum hexaboride (LaB6)) is an inorganic non-metallic compound composed of low-valence boron and the rare metal element lanthanum. The crystal structure of LaB6 is simple cubic space group.
LaB₆ is Calcium hexaboride structured and crystallizes in the cubic Pm̅3m space group. La is bonded in a 24-coordinate geometry to twenty-four equivalent B atoms. All La-B bond lengths are 3.05 Å. B is bonded in a 5-coordinate geometry to four equivalent La and five equivalent B atoms. There is one shorter (1.66 Å) and four longer (1.76 Å) B-B bond lengths.
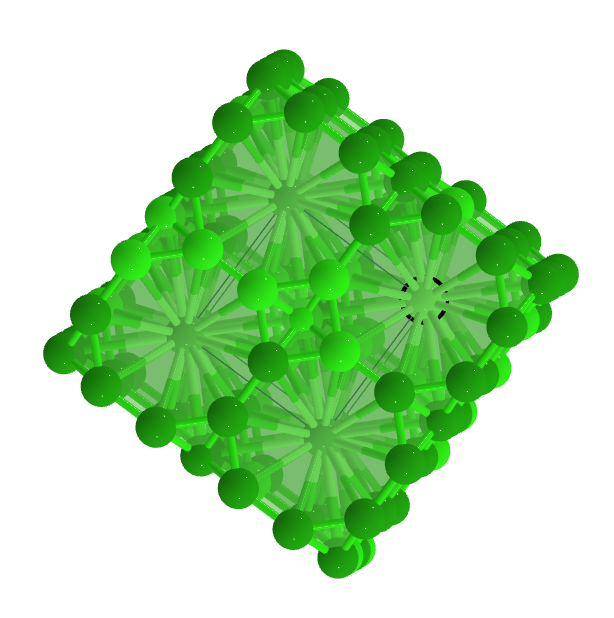
Lanthanum hexaboride stands out for its crystal structure. Inside the cubic lattice, the lanthanum atom locates at the center, while eight octahedral boron atoms sit symmetrically in the corner of the cube and surround the metal atom. There is no valence bond between the metal atom and the boron atoms, while the boron atoms are bonded to each other.
Lanthanum hexaboride is notable for its thermionic emission and mechanical strength and is being explored for its potential applications in IR-absorbing photovoltaic cells and thermally insulating window coatings. Its single crystal is used to make high-power electron tube.
You may like
See also
Lastest Price from LANTHANUM BORIDE manufacturers
US $0.00/kg2022-09-28
- CAS:
- 12008-21-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $15.00-10.00/KG2021-08-12
- CAS:
- 12008-21-8
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton