What is the crystal structure of beryllium hexaboride?
Beryllium hexaboride (BeB6) is a compound with unique properties that can be used for a variety of applications. It is known for its ultra-hardness and superconductivity, properties that contribute to the development of advanced materials with tunable multifunctional properties.
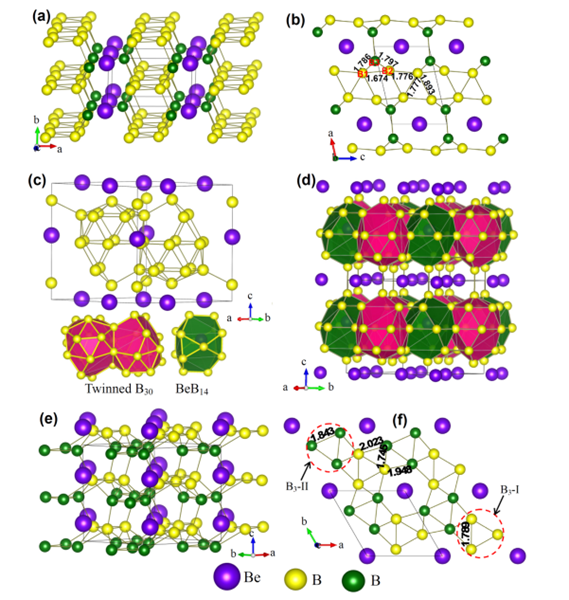
BeB6 has three different crystal structures, α-BeB6, β-BeB6, and γ-BeB6, which are stable at both atmospheric and high pressures. The α-BeB6 structure features rhomboid B4 units, which are interconnected to extended B∞ ribbons and intercalated by beryllium (Figure a,b).
In BeB6, the Be atom in fact participates in the (partially covalent) Be−B bonding rather than severing as an electron donor, which dictates that the structure of BeB6 differs from other Zintl−Klemm compounds. The rhomboid B4 in αBeB6 has two distinct edge lengths, 1.776 and 1.893 Å, while neighboring units are connected by a shorter bond of 1.674 Å. This range of bond lengths is comparable to that of α-B (1.669−2.003 Å) and γ-B (1.661−1.903 Å).
The β-BeB6 features cage-like boron slabs intercalated by beryllium (Figure c,d). The B−B bond lengths in the slab range from 1.740 to 1.977 Å, and within the interslabs, the B−B bonds are varied as well (1.800, 1.805, and 1.892 Å), while the Be−B bond lengths change from 1.952 to2.203 Å, slightly greater than those in the α-BeB6.
The γ-BeB6 (Figure e,f) features buckled boron sheets similar to the 2D boron allotrope. The formation of these sheets is a result of competition between two- and three-center bonding, which induces a B3 isosceles triangle (with bond lengths of 1.789 and 1.843 Å) connected by distorted B4 rhomboids (with bond lengths of 1.745−2.023 Å). Remarkably, the shortest Be−B bond length in γ-BeB6 is 1.790 Å, much shorter than those in αBeB6 and β-BeB6. This bond length is between typical single (1.87 Å) and double (1.68 Å) Be−B covalent length, implying the relatively strong Be−B bonding in γ-BeB6.
References:
[1] LAILEI WU. Coexistence of Superconductivity and Superhardness in Beryllium Hexaboride Driven by Inherent Multicenter Bonding.[J]. The Journal of Physical Chemistry Letters, 2016. DOI:10.1021/ACS.JPCLETT.6B02444.