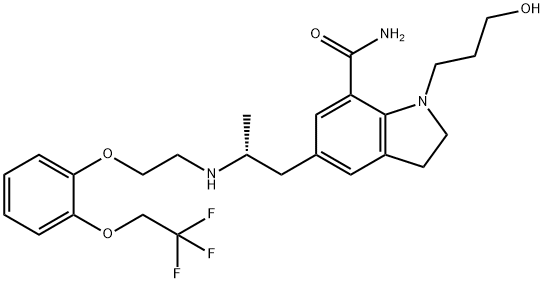
What is Silodosin?
General description
Silodosin is a selective antagonist of alpha(α)-1 adrenergic receptors that binds to the α1A subtype with the highest affinity. α1-adrenergic receptors regulate smooth muscle tone in the bladder neck, prostate, and prostatic urethra: the α1A subtype accounts for approximately 75% of α1-adrenoceptors in the prostate (1,2).
Silodosin was first approved by the FDA in October 2008 (2) and it is also approved in Europe and Canada. Silodosin is available as oral capsules with common trade names Rapaflo and Urorec. It is indicated for the symptomatic treatment of benign prostatic hyperplasia in adults (7). Most commonly affecting males over the age of 40 years, benign prostatic hyperplasia is the non-malignant enlargement of the prostate gland, associated with lower urinary tract symptoms that have a negative impact on the quality of life of patients (2). Silodosin works by binding to α1A-adrenoceptors with high affinity and relaxing the lower urinary tract, thereby improving urinary symptoms and alleviating bladder outlet obstruction (6).
Figure 2 Silodosin capsules
Indications
Silodosin is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH). It is not indicated for the treatment of hypertension (7).
Pharmacodynamics
Silodosin is an antagonist of α1-adrenoceptors. It has the highest selectivity for the α1A-adrenoceptor subtype, with a 162-fold greater affinity than α1B-adrenoceptor and about a 50-fold greater affinity than for α1D-adrenoceptor. In clinical trials, silodosin improved maximum urinary flow rate, voiding symptoms, and storage symptoms of benign prostatic hyperplasia (2-4). Following oral administration, silodosin had a rapid onset of effect in men 3, with early effects of relieving lower urinary tract symptoms occurring within two to six hours post-dose (6).
Silodosin inhibited the human ether-a-go-go-related gene (HERG) tail current; however, it has weak cardiovascular effect (5). As with all α1-adrenoceptor antagonists blocking α1-adrenoceptors in the iris dilator muscle, silodosin may cause intraoperative floppy iris syndrome (IFIS), which is characterized by small pupils and iris billowing during cataract surgery in patients taking α1-AR antagonists (6).
Silodosin improved bladder urodynamic parameters in men with BPH [7, 8]. Silodosin 4 mg twice daily was associated with significant (p<0.001) reductions from baseline in detrusor opening pressure [7], detrusor pressure at maximum urinary flow rate (Qmax) [7, 8] and the bladder outlet obstruction index [7, 8]. Silodosin recipients had a significant (p <0.01) increase from baseline in the maximum cytometric capacity in one study [13] and in the bladder capacity at first void in a second study [8]. The majority of patients (>75 %) with detrusor over-activity at baseline experienced resolution or improvement of detrusor over-activity with silodosin therapy [7, 8]. Voiding and storage symptoms and Qmax were improved with silodosin in men with LUTS associated with BPH.
In keeping with its selectivity for a1A-adrenoceptors over a1B-adrenoceptors (which help maintain vascular smooth muscle tone), silodosin was associated with a low risk of orthostatic hypotension in men with LUTS associated with BPH. In men with BPH who received silodosin 4 or 8 mg/day, positive orthostatic tests occurred in 4.5 and 3.3 % of patients, respectively [7].
In healthy men, therapeutic (8 mg/day) and supratherapeutic (24 mg/day) dosages of silodosin were not associated with prolongation of the heart rate-corrected QT (QTc) interval, according to the results of a thorough QT study [7].
Mechanism of action
The pathogenesis of benign prostatic hyperplasia is not fully understood: it is believed to involve several pathways, including inflammation, apoptosis, and cellular proliferation. Most drug therapies aim to alleviate symptoms of benign prostatic hyperplasia, silodosin included. Lower urinary tract symptoms of benign prostatic hyperplasia are categorized into three main groups: voiding or obstructive (hesitancy, slow stream, intermittency, incomplete emptying), storage or irritative (frequency, urgency, nocturia, urge urinary incontinence), and postmicturition (postvoid dribbling). Prostate contraction is the main contributor to lower urinary tract symptoms of benign prostatic hyperplasia. The smooth muscle tone of the prostate is regulated by α1A-adrenoceptors, which are the most highly expressed subtype of α1adrenoceptors in the human prostate tissue (2). It has been reported that blockade of α1A-adrenoceptors relieves bladder outlet obstruction. Blockade of α1D-adrenoceptors, another subtype found in prostate tissue, is believed to alleviate storage symptoms due to detrusor overactivity (6).
α1-adrenoceptors are G protein-coupled receptors: upon binding of its natural ligand, norepinephrine and epinephrine, leads to the activation of phospholipase C and downstream signaling molecules, including inositol triphosphate and diacylglycerol. Ultimately, there is an increase in intracellular calcium levels and, consequently, smooth muscle contraction. Silodosin is an antagonist of α1-adrenoceptors, with the highest selectivity for the α1A-adrenoceptor subtype. By blocking the α1A-adrenoceptor signaling pathway, silodosin promotes prostatic and urethral smooth muscle relaxation, thereby improving lower urinary tract symptoms such as voiding. Silodosin also targets afferent nerves in the bladder, relieving bladder overactivity and storage symptoms (2).
Dosage and administration
Silodosin is approved in the USA and the EU for the treatment of the signs and symptoms of BPH, and in Japan for the treatment of bladder outlet obstruction associated with BPH [9]. The recommended silodosin dosage is 8 mg once daily in the USA and the EU and 4 mg twice daily in Japan [9].
Local prescribing information should be consulted for more information regarding contraindications, warnings and precautions associated with silodosin.
Toxicity
In clinical trials, postural hypotension was the most common dose-limiting adverse event. In case of drug overdose leading to hypotension, the patient should be placed in a supine position to restore blood pressure and normalize heart rate. Further measures, such as administration of intravenous fluids, may be initiated. In case of the use of vasopressors, renal function should be monitored and supported as needed. Since silodosin is highly bound to plasma proteins, dialysis is unlikely to be beneficial (9).
Preparation
During the preparation of silodosin using the patented route (Scheme 1), four key intermediates were observed: IM-A and IM-B are starting materials for the synthesis of SI-III, IM-C is the disubstituted by-product from SI-III, and IM-D is the S-enantiomer of silodosin (Figure 1) (10). SI-III is reacted with oxalic acid to get SI-IV, which is further undergoes NaOH reaction to generate the SI-V. The later further react with H2O2 and NaOH to obtain the final product of silodosin.


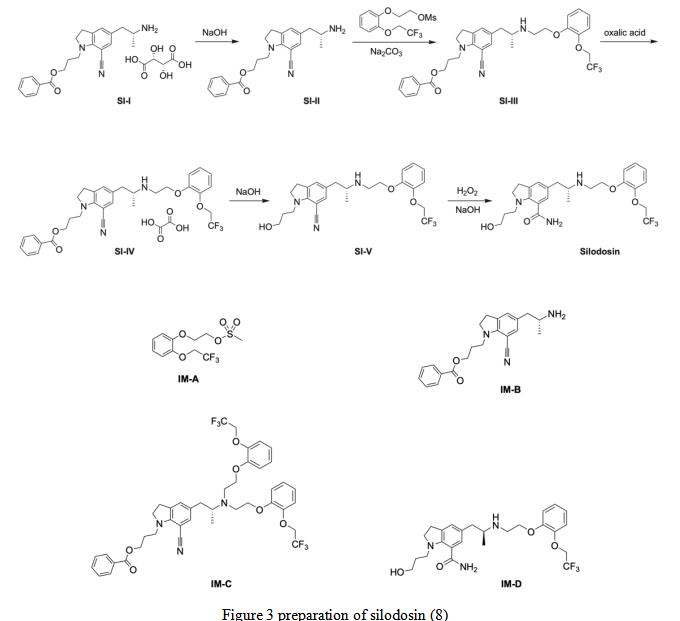
Figure 3 preparation of silodosin (8)
References
1. Yoshida M, Homma Y, Kawabe K: Silodosin, a novel selective alpha 1A-adrenoceptor selective antagonist for the treatment of benign prostatic hyperplasia. Expert Opin Investig Drugs. 2007 Dec;16(12):1955-65.
2. Rossi M, Roumeguere T: Silodosin in the treatment of benign prostatic hyperplasia. Drug Des Devel Ther. 2010; 4: 291-7.
3. Keating GM: Silodosin: a review of its use in the treatment of the signs and symptoms of benign prostatic hyperplasia. Drugs. 2015 Feb;75(2):207-17.
4. Vishnuvardhan C, Baikadi S, Borkar RM,4. Srinivas R, Satheeshkumar N: In vivo metabolic investigation of silodosin using UHPLC-QTOF-MS/MS and in silico toxicological screening of its metabolites. J Mass Spectrom. 2016 Oct;51(10):867-882.
5. Tatemichi S, Kiguchi S, Kobayashi M, Yamazaki Y, Shibata N, Uruno T: Cardiovascular effects of the selective alphalA-adrenoceptor antagonist silodosin (KMD-3213), a drug for the treatment of voiding dysfunction. Arzneimittelforschung. 2006;56(10):682-7.
6. Yamanishi T, Mizuno T, Kamai T, Yoshida K, Sakakibara R, Uchiyama T: Management of benign prostatic hyperplasia with silodosin. Open Access J Urol. 2009 Aug 20; 1: 1-7.
7. Yamanishi T, Mizuno T, Tatsumiya K, et al. Urodynamic effects of silodosin, a new a1A-adrenoceptor selective antagonist, for the treatment of benign prostatic hyperplasia. Neurourol Urodyn. 2010;29(4):558–62.
8. Matsukawa Y, Gotoh M, Komatsu T, et al. Efficacy of silodosin for relieving benign prostatic obstruction: prospective pressure flow study. J Urol. 2009;182(6):2831–5.
9. Watson Pharma Inc. RapafloÒ (silodosin) capsules: US pre- scribing information. 2013. http://www.actavis.com/. Accessed 8 Dec 2014.
10. FDA Approved Drug Products: RAPAFLO (silodosin) Capsule for oral use.
11. Hui Li et al Improved Preparation of Silodosin. The New Journal for Organic Synthesis Volume 51, 2019 - Issue 3
You may like
Related articles And Qustion
Lastest Price from Silodosin manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 160970-54-7
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $100.00-75.00/kg2025-04-21
- CAS:
- 160970-54-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton