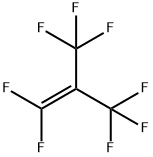
What is Perfiuoroisobutylene?
Perfluoroisobutene (PFIB) is the perfluorocarbon counterpart of the hydrocarbon isobutene and has the formula (CF3)2C=CF2. An alkene, it is a colorless gas that is notable as a highly toxic perfluoroalkene. Few simple alkenes are as toxic.
Perfluoroisobutylene (PFIB) is a schedule 2A substance under the Chemical Weapons Convention, which means that while it has significant ability to be used as a chemical weapon, it also serves various other industrial uses.
Uses
Perfluoroisobutylene or perfluoroisobutene is a monomer used in synthesis of Teflon and other polymeric materials. It is also used in etching for semiconductor fabrication, and is potentially used as a chemical warfare agent. The US Food and Drug Administration’s CFR 21 Section 173.360 allows for use of octafluorocyclobutane as a propellant and also allows for PFIB at a level of <.01% as an impurity in formulation.
Mechanism of Toxicity
PFIB is a strong electrophile that reacts with nucleophiles. The
toxicity of PFIB may be correlated with its susceptibility to
nucleophilic attack and the generation of reactive
intermediates.
Safety
Perfluoroisobutene is quite toxic with an LCt = 880 mg⋅min⋅m−3 (mice).[1] It is a Schedule 2 substance of the Chemical Weapons Convention. Perfluoroisobutene is highly reactive toward nucleophiles. It hydrolyzes readily to give the relatively innocuous (CF3)2CHCO2H, which readily decarboxylates to give hexafluoropropane. It forms addition compounds with thiols, and it is this reactivity that may be related to its toxicity.[1] PFIB is a product of pyrolysis of polytetrafluoroethylene (PTFE), one of the substances invoked to explain polymer fume fever.
Environmental Fate
PFIB exists as a gas in the atmosphere, and is degraded by
reaction with hydroxyl radicals, with a reaction half-life
of ~5.7 days. PFIB is not susceptible to significant photolysis.
The Henry’s law constant of PFIB suggests volatization as
an important fate process. The half lives for volatization
calculated from a model lake and river were 5.6 days and
4.1 h, respectively, though a small portion will adsorb to
suspended solids and sediment. PFIB can also volatize
substantially from moist soils, and to a small degree from dry
soils.