What is Oritavancin?
DESCRIPTION
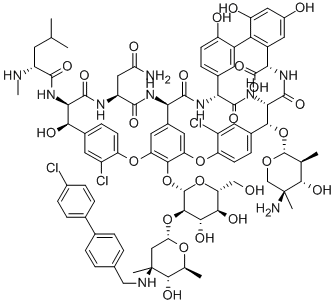
Oritavancin is a second-generation lipoglycopeptide antibiotic derived from modifications of chloroeremomycin, a naturally occurring glycopeptide. Structurally, oritavancin is very similar to vancomycin. The only difference is the addition of an aromatic lipophilic side chain and an unsubstituted sugar. The resulting molecule possesses both hydrophilic and hydrophobic regions. These side chains not only increase oritavancin’s spectrum of activity, but also increase its half-life. Oritavancin is effective against Gram-positive aerobes, including vancomycin-resistant organisms, such as vancomycin-resistant Enterococcus (VRE) and Staphylococcus aureus isolates that are vancomycin resistant (vancomycin-resistant S. aureus, VRSA) or vancomycin intermediate-resistant (vancomycin intermediate-resistant S. aureus, VISA). It also possesses activity against a number of Gram-positive anaerobic bacteria.
Mechanistically, oritavancin exerts its action by interfering with bacterial cell wall synthesis and altering membrane potential. This is accomplished by binding to the D-alanyl-D-alanine (D-Ala-D-Ala) terminus of the peptidoglycan chain in the target bacterium.
MECHANISM OF DRUG ACTION
Oritavancin exerts its antibacterial actions in two ways. The first mechanism of action, not unlike the other glycopeptide antibiotics, is through inhibition of cell wall synthesis. During the synthesis process, peptidoglycan precursors are formed by the active bacteria and incorporated into their growing cell wall. It is with the latter step that oritavancin interferes. It does so by the formation of a complex with the D-Ala-D-Ala terminus of the developing peptidoglycan chain. This action is very similar to that of vancomycin. However, oritavancin’s affinity for the peptidoglycan terminus far exceeds that of vancomycin; especially for VRE and resistant S. aureus. One possible explanation for this increased binding affinity is oritavancin’s ability to form a homodimer prior to binding. Each dimer then has the ability to interact with two developing peptidoglycan precursors instead of the single one afforded by a lone molecule. This, in turn, creates an extra binding site to the cytoplasmic membrane. Each site is consumated by the attachment of the D-Ala-D-Ala residue via undecaprenyl pyrophosphate. In addition, the p-chlorophenylbenzyl side chains of the dimer molecules also form a bond with the membrane, thus positioning the antibiotic for peptidoglycan interactions. Alternatively, it has been proposed that the activity of oritavancin is the result of a secondary binding action. This may explain why electron microscopy has shown that oritavancin but not vancomycin induced aberrant septum formation both in S. aureus and vancomycin resistant enterococcus. Oritavancin’s second mechanism of action is caused by these aforementioned side chains binding to the cell membrane and causing rapid changes in membrane potential and ultimately membrane permeability. This may be due to interactions of oritavancin with phospholipids bilayers and thereby changes in lipid organization. As a result of this dual mechanism of action oritavancin, unlike vancomycin, displays rapid bactericidal activity. This mechanism has also been postulated to explain the activity of oritavancin against S. aureus in several phases of growth, including in stationary-phase and biofilms.
TOXICITY
The safety of oritavancin has been reported in one of two phase II trials and two phase III trials. In each case, the comparator medication was vancomycin. With respect to the phase III trials, vancomycin was followed by oral cephalexin. The most common adverse effects reported in the phase II trial were similar to those of the comparator and included injection site phlebitis (19%), fever (19%), diarrhea (17%), and hypotension (16%) (Loutit et al., 2004). Adverse effects reported from the first phase III trial (n = 517) were found to be similar to vancomycin, with phlebitis, nausea and vomiting, and pruritus being the most common. The second (n = 1246) found significantly fewer adverse effects reported in the oritavancin group than in the vancomycin group.
In addition to the patient trials, safety was also reported in two healthy volunteer studies. In both instances, reactions most commonly reported were mild and included administration site conditions, headache, rhinitis, dry skin, and pain. There were five reports of transient elevations in aspartate transaminase (AST) and/or alanine aminotransaminase (ALT). Rises in four of the five subjects were less than three times the upper limit of normal and returned to baseline within 6 days. Interpretation of the remaining subject’s results was complicated by admitted alcohol consumption. In addition, there was one report of injection site thrombosis.
You may like
Lastest Price from ORITAVANCIN manufacturers

US $5.00-0.50/KG2025-05-29
- CAS:
- 171099-57-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $79.00-38.00/kg2025-04-21
- CAS:
- 171099-57-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton