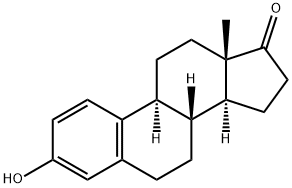
What is Estrone?
Estrone, one of the major mammalian estrogens, is an aromatized C18 steroid with a 3-hydroxyl group and a 17-ketone. It is produced in vivo from androstenedione or from testosterone via estradiol. It is produced primarily in the ovaries, placenta, and in peripheral tissues (especially adipose tissue) through conversion of adrostenedione. Estrone may be further metabolized to 16-alpha-hydroxyestrone, which may be reduced to estriol by estradiol dehydrogenase.
Mechanism of action
Estrogens enter the cells of responsive tissues (e.g. female organs, breasts, hypothalamus, pituitary) where they interact with estrogen receptors. Hormone-bound estrogen receptors dimerize, translocate to the nucleus of cells and bind to estrogen response elements (ERE) of genes. Binding to ERE alters the transcription rate of affected genes. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) release from the anterior pituitary.
Synthesis Method
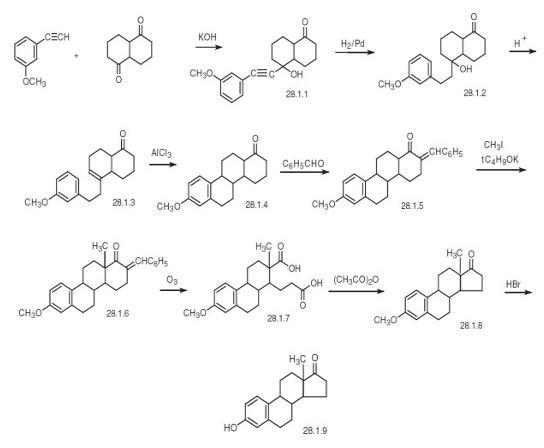
Estrone, 3-hydroxyestra-1,3,5(10)-trien-17-one (28.1.9), has been made synthetically in various ways. According to one of the first and most simple schemes, synthesis was carried out in the following manner. Condensation of 3-methoxyphenylacetylene with bicyclohexane-1,5-dione in a Favorskii reaction conditions lead to the corresponding carbinol (28.1.1). The triple bond was reduced by hydrogen over a palladium catalyst, forming the tertiary alcohol (28.1.2), which was then dehydrated in acidic conditions to give the compound (28.1.3). Intramolecular alkylation of this compound in the presence of anhydrous aluminum chloride formed a tetracyclic ketone (28.1.4), which during condensation with benzaldehyde was transformed into an eneone (28.1.5). This was methylated at the β-position relative to the keto-group by methyl iodide in the presence of potassium tert-butylate, and the resulting compound (28.1.6) underwent ozonolysis, forming the dicarboxylic acid (28.1.7). Cyclization of this compound to a cyclopentanone derivative lead to the formation of methyl ester of the desired estrone (28.1.8), and demethylation of the phenolic hydroxyl group by hydrobromic acid formed the desired estrone (28.1.9).
You may like
Related articles And Qustion
Lastest Price from Estrone manufacturers

US $150.00/g2025-11-24
- CAS:
- 53-16-7
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 999

US $150.00-850.00/G2025-07-10
- CAS:
- 53-16-7
- Min. Order:
- 100G
- Purity:
- 99
- Supply Ability:
- 9999