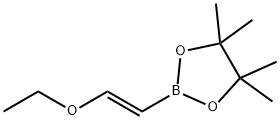
What is (E)-1-Ethoxyethene-2-ylboronic acid pinacol ester?
(E)-1-Ethoxyethene-2-boronic acid pinacol ester ((E)-2-(2-Ethoxy-vinyl)-boronic acid pinacol ester; (E)-(2-Ethoxyvinyl)boronic acid, pinacol ester 98%; (E)-1-Ethoxyethene-2-ylboronic acid pinacol ester; (E)-1-Ethoxyethene-2-boronic acid pinacol ester; 1,3,2-Dioxaboro,2-[(1E)-2-ethoxyethenyl]-4,4,5,5-tetraMethyl-; trans-2-Ethoxyvinyl boronic acid pinacol ester 97%; (E)-2-(2-Ethoxyvinyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; (E)-2-Ethoxyvinyl boronic acid pinacol ester; C10H21BO4) as chiral pinacol vinylboronate is a boronic ester used in Suzuki-Miyaura cross-coupling reactions. Thus, it can be used to synthesize azaindole and diazaindoles from chloroamino-N-heterocycles.
Pinacol vinylether boronate ((E)-1-Ethoxyethene-2-boronic acid pinacol ester) could be synthesized based on the reaction between boronic acid pinacol ester and ethoxy acetylene in present of organometallic complex catalyst of Cp2ZrCl(H) [2]. However, the highly flammable ethoxy acetylene was not suitable to be used on an industrial scale due to latent tremendous safety concerns in industrial production. Pinacol vinylether boronate also could be prepared by a dehydrogenative borylation of alkenes, which would consist of a catalytic (rhodium, titanium or ruthenium) hydroboration of ethoxyethene with boronic acid pinacol ester, followed by the elimination of hydrogen [3]. It should be mentioned that the desired pinacol vinylether boronate was formed almost exclusively without competing hydroboration or hydrogenation of the vinylboronate in present of certain rhodium-, ruthenium- or palladium catalysts.

Pinacol vinylether boronate is an important synthetic reagent in used in Suzuki-Miyaura cross-coupling reactions. For example, in synthesis process of the propane-1-sulfonic acid {3-[5-(4-chloro-phenyl)-1H-pyrrolo [2,3-b] pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide, the key reaction step according to the previous reports, namely the reactions for preparing 5-(4-Cl-phenyl)-7-azaindole, have been accomplished via the Suzuki-Miyaura reaction with (E)-1-Ethoxyethene-2-boronic acid pinacol ester [4]. This reaction could be occurred under basic conditions (pH-values above 7, N(CH2CH3)3, Na2CO3, LiOH and the like) in organic solvents (toluene, dimethylformamide, or mixtures of dioxane and water) or mixtures of organic solvents with water, and in the presence of palladium (Pd) catalysts (PdCl2(dppf)CH2Cl2, Pd(OAc)2 and the like). Moreover, by the Suzuki-Miyaura cross-coupling reactions, 4-chloro-6-cyclohexyl-2-methylthieno[2,3-d]pyrimidine in DMF could react with (E)-1-ethoxyethene-2-boronic acid pinacol ester in present of K3PO4 and Pd(PPh3)4 under an argon atmosphere to obtain 6-cyclohexyl-4-[(E)-2-ethoxyvinyl]-2-methylthieno [2,3-d] pyrimidine [5]. Ethyl 5-bromo-2,4-dimethyl-thieno [2,3-d] pyrimidine-6-carboxylate and (E)-1-ethoxyethene-2-boronic acid pinacol ester also could occurred Suzuki-Miyaura reaction in mixture of 1,4-dioxane and water with the [1,1]’-bis(diphenyl phosphino) ferrocene] dichloro palladium and cesium carbonate catalysts [6]. After diluted with EtOAc, washed with H2O and brine, dried over Na2SO4, filtered, concentrated, and purified using flash column chromatography on silica gel, a pale yellow solid of the ethyl 5-[(E)-2-ethoxyvinyl]-2,4-dimethyl-thieno [2,3-d] pyrimidine-6-carboxylate would be obatained.
In conclusion, pinacol vinylether boronate ((E)-1-Ethoxyethene-2-boronic acid pinacol ester) is important intermediate participating Suzuki-Miyaura cross-coupling reactions for preparing bicyclic compounds.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB32598256.htm.
[2] Satoh, M., Miyaura, N., & Suzuki, A. (1987). Palladium-catalyzed cross-coupling reaction of (1-ethoxy-1-alken-2-yl) boranes with ortho-functionalized iodoarenes. A novel and convenient synthesis of benzo-fused heteroaromatic compounds. Synthesis, 1987(04), 373-377.
[3] Brumsted, C. J., Moorlag, H., Radinov, R. N., Ren, Y., & Waldmeier, P. (2014). U.S. Patent No. 8,779,150. Washington, DC: U.S. Patent and Trademark Office.
[4] Brumsted, C. J., Moorlag, H., Radinov, R. N., Ren, Y., & Waldmeier, P. (2017). U.S. Patent No. 9,556,172. Washington, DC: U.S. Patent and Trademark Office.
[5] https://pubchem.ncbi.nlm.nih.gov/compound/21973908
[6] http://www.chemspider.com/Chemical-Structure.10728996.html?rid=9b2796e0-4c22-4c15-a2d5-4f6c9ed18269
See also
Lastest Price from (E)-1-Ethoxyethene-2-ylboronic acid pinacol ester manufacturers

US $3.00-9.00/KG2025-07-02
- CAS:
- 1201905-61-4
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available