What is an Azelastine hydrochloride nasal solution?
Introduction
Azelastine hydrochloride is an intranasal antihistamine indicated for use in patients with seasonal allergic rhinitis (SAR) and nonallergic vasomotor rhinitis (VMR). Up to 20% of the US population, or approximately 60 million people, are thought to suffer from some form of chronic rhinitis.

The approval of Azelastine in 1996 for allergic rhinitis and subsequently in 1999 for nonallergic rhinitis has expanded the therapeutic options available for treating both allergic and nonallergic rhinitis. The importance of this medication for the treatment of chronic rhinitis is supported by the positioning of azelastine HCl as a first-line treatment for allergic rhinitis (AR) and nonallergic rhinitis (NAR) by the World Health Organization and Joint Task Force of the American Academy of Allergy, Asthma and Immunology. Its multi-mechanistic actions and effectiveness across the range of chronic rhinitis subtypes have significantly impacted the health of a vast number of patients suffering from chronic rhinitis[1].
Chemistry property
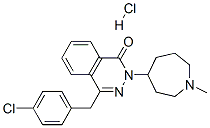
Azelastine is a white, nearly odourless crystal with a distinct bitter taste. The molecule is soluble in dichloromethane and chloroform, sparingly soluble in propylene glycol and methanol, slightly soluble in glycerin, octanol, and ethanol, but practically insoluble in hexane. The chemical name of azelastine hydrochloride is (±)-1-(2H)-phthalazinone,4-[(4- chlorophenyl) methyl]-2-(hexahydro-1-methyl1H-azepin-4-yl)-monohydrochloride, the molecular weight is 418.37, and the empirical formula is C22H24ClN3 O•HCl. Azelastine hydrochloride is a bitter-tasting, white powder that is nearly odourless.
Uses
Azelastine hydrochloride (Astelin) nasal spray 0.1% solution is a second-generation intranasal antihistamine available in the United States for the treatment of both seasonal allergic rhinitis (SAR) and nonallergic vasomotor rhinitis (VMR). A 0.05% ophthalmic preparation of azelastine hydrochloride (Optivar) is sold in the US to treat ocular itching associated with allergic conjunctivitis. Azelastine nasal spray was first marketed in the United Kingdom in 1991 for treating both SAR and perennial allergic rhinitis (PAR) and is currently marketed in approximately 80 countries under various proprietary names. Oral formulations are approved for treating allergic rhinitis, asthma, and urticaria in several Asian nations. Research programs involving azelastine hydrochloride are currently being carried out in the US to develop new anti-allergy products that take advantage of the known pharmacologic properties of the molecule.
Azelastine hydrochloride nasal solution contains 0.1% azelastine hydrochloride in an aqueous solution at pH 6.8 ± 0.3. It also contains benzalkonium chloride (125 mcg/mL), citric acid, dibasic sodium phosphate, edetate disodium, hypromellose, purified water and sodium chloride. After priming, each metered spray delivers a 0.137 mL mean volume containing 137 mcg of azelastine hydrochloride (equivalent to 125 mcg of azelastine base). The bottle can deliver 200 metered sprays.
Side effects
In United States registration trials for SAR, treatment with azelastine nasal spray was well tolerated for treatment durations up to 4 weeks in adults and children 12 years and older. Bitter taste, headache, somnolence, and nasal burning were the most frequently reported adverse events, but most cases were mild or moderate[2].
The most common adverse effect of azelastine nasal spray is a bitter taste; however, this problem can be reduced by using the dosing technique recommended in the product labelling from the manufacturer. This involves tilting the head slightly forward and not inhaling the medication deeply to avoid deposition in the nasopharynx. Adverse experience information for the azelastine one spray per nostril twice daily dosage is derived from two placebo-controlled, 2-week clinical studies that included 276 patients. None of the patients receiving Azelastine nasal spray were discontinued from these studies due to adverse reactions.
[1] Horbal, J. and J. Bernstein. “Azelastine HCl: A Review of the Old and New Formulations.” 2010. 0.
[2] Bernstein, Jonathan A. “Azelastine hydrochloride: a review of pharmacology, pharmacokinetics, clinical efficacy and tolerability.” Current Medical Research and Opinion 23 10 (2007): 2441–52.
References:
[1] HORBAL J, BERNSTEIN J. Azelastine HCl: A Review of the Old and New Formulations[C]. 2010. DOI:10.4137/CMT.S3865.[2] BERNSTEIN J A. Azelastine hydrochloride: a review of pharmacology, pharmacokinetics, clinical efficacy and tolerability.[J]. Current Medical Research and Opinion, 2007, 23 10. DOI:10.1185/030079907X226302.
Lastest Price from Azelastine hydrochloride manufacturers

US $0.00/g2025-09-22
- CAS:
- 79307-93-0
- Min. Order:
- 100g
- Purity:
- 99%min
- Supply Ability:
- 300kg/month

US $1.00-4.00/KG2025-05-14
- CAS:
- 79307-93-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG