What are the uses and synthesis routes of 1,5-Pentanediol?
1,5-Pentanediol (1,5-PDO) is a linear diol with an odd number of methylene groups. It is an important raw material for the synthesis of polyesters, unsaturated polyesters and polyurethanes. It can also be used to produce plastics, cosmetics and food. In addition, studies have shown that 1,5-PDO has antibacterial and antifungal properties and can be used to treat hair loss, cold sores, nail problems, dry and scaly feet, and eczema; it can also be used as a moisturizer and solvent.

Synthesis method
Currently, 1,5-Pentanediol is mainly produced by chemical processes using dimethyl glutarate as a raw material. It can also be obtained by hydrogenating tetrahydropyran-2-yl acetate through esterification.
1,5-PDO has also been produced by chemical catalytic processes using furfural and tetrahydrofurfuryl alcohol materials extracted from biomass. However, these processes have problems such as high cost, cumbersome steps and environmental safety. Therefore, it is important to develop a bioprocess for the production of 1,5-PDO, i.e., to form a cell factory in E. coli to produce 1,5-PDO.
Method 1: Chemical synthesis
A new process for the production of 1,5-PDO using 3,4-dihydro-2H-pyran (DHP) and acetic acid (AA) as raw materials.
Steps: The esterification reaction of DHP and AA was carried out at 373 K for 1 hour with a DHP/AA molar ratio of 1. Under catalyst-free conditions, the DHP conversion was 59.8% and the selectivity of tetrahydropyran-2-yl acetate (THPOAc) was 91.2%. Then, THPOAc was hydrogenated over a Cu/Zn/Al catalyst at 453 K and 50 bar to obtain 1,5-PDO with a selectivity of 54.5%. The higher the dispersion of Cu nanoparticles, the larger the surface area of metallic Cu, the more Cu active sites, and the lower the acidity of the Cu/Zn/Al catalyst, the more favorable the hydrogenation of THPOAc to 1,5-PDO.
Method 2: Chemical catalysis
Synthesis of 1,5-pentanediol from tetrahydrofurfuryl alcohol by hydrogenolysis. To develop an efficient method for the production of 1,5-PDO, a series of MgAl2O4-modified Pt/WOx/γ-Al2O3 catalysts with different compositions were prepared by impregnation-calcination. The physicochemical properties of these catalysts were then characterized using different techniques. The characterization showed that the magnesium aluminum spinel modification enhanced the dispersion of Pt particles, the adsorption of CO on Pt/WOx/γ-Al2O3, reduced the reduction temperature of Pt particles, reduced the acid content in the catalyst, and increased the surface oxygen vacancy concentration. These changes appear to affect the performance of the catalyst, but other factors cannot be ruled out. Catalytic activity tests showed that magnesium aluminum spinel modification improved the tetrahydrofurfuryl alcohol hydrogenolysis activity and 1,5-pentanediol selectivity of Pt/WOx/γ-Al2O3. The best performance was achieved when the magnesium aluminum spinel loading was 12%, with a tetrahydrofurfuryl alcohol conversion of 47.3% and a 1,5-pentanediol selectivity of 88.4%.
Method 3: Biosynthesis
The biosynthetic pathway of the artificial pathway for the biosynthesis of 1,5-PDO from lysine is shown in Fig 1. below, which has a comprehensive cofactor and cosubstrate cycle.
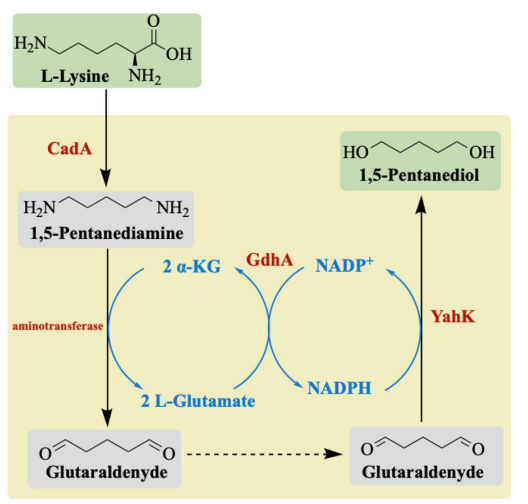
Steps: The pathway from lysine to 1,5-PDO uses three enzymes: lysine decarboxylase, aminotransferase, and alcohol dehydrogenase. Aminotransferases from different organisms were first screened to identify an enzyme that can successfully transfer the two amines in cadaverine, thereby identifying GabT in Escherichia coli. It was then cascaded with lysine decarboxylase and alcohol dehydrogenase in Escherichia coli to achieve whole-cell production of 1,5-PDO from lysine. To improve the whole-cell activity of 1,5-PDO production, we used the EutM protein scaffold for GabT assembly and verified the recycling of NADPH and α-ketoglutarate (α-KG) by glutamate dehydrogenase. After optimizing the culture and bioconversion conditions, the titer of 1,5-PDO reached 4.03 mM.
References:
[1] WENFENG HUA. An integrated cofactor and co-substrate recycling pathway for the biosynthesis of 1,5-pentanediol.[J]. Microbial Cell Factories, 2024, 23 1: 132. DOI:10.1186/s12934-024-02408-y.
[2] ZHAO S, DU H, FANG Q, et al. 1,5-Pentanediol production from 3,4-dihydro-2H-pyran and acetic acid via successive reactions of esterification and hydrogenation[J]. Green Synthesis and Catalysis, 2024, 143 1. DOI:10.1016/j.gresc.2024.02.005.
[3] WANG W, CHEN C. Selective Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol over MgAl2O4-Modified Pt/WO3/γ-Al2O3 Catalyst[J]. Catalysts, 2024, 174. DOI:10.3390/catal14070428.
Lastest Price from 1,5-Pentanediol manufacturers
US $1.50/g2025-06-24
- CAS:
- 111-29-5
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons

US $10.00/kg2025-04-21
- CAS:
- 111-29-5
- Min. Order:
- 200kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/day


