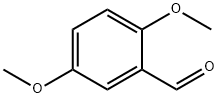
What are the Steps in the Synthesis of 2,5-Dimethoxybenzaldehyde?
2,5-Dimethoxybenzaldehyde synthesis: Anethole is oxidized to anisaldehyde , which after isolation is subjected to a BaeyerVilliger oxidation reaction with performic or peracetic acid. The O-formyl-4-methoxyphenol obtained this way is hydrolyzed . 4-Methoxyphenol is subsequently formylated using the Reimer-Tiemann method and the obtained 2-hydroxy-5-methoxybenzaldehyde is methylated with dimethylsulfate to 2,5-dimethoxybenzaldehyde.
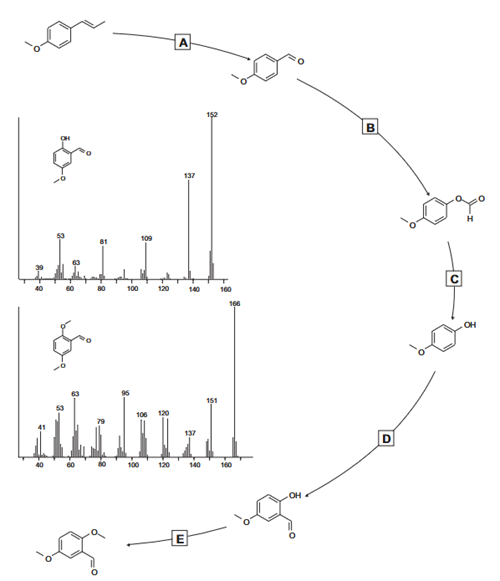
The specific steps are as follows:
A : Anisaldehyde from anethole via oxidative cleavage: 20 g anise oil was suspended in a mixture of 150 mL water and 30 mL conc. sulfuric acid; addition of 55 g sodium bichromate at such a rate that the temperature did not exceed 40°C. The reaction mixture was extracted with 4 x 125 mL toluene and the solvent evaporated. The residual oil was vacuum distilled to yield 9.1 g anisaldehyde.
B : O-formyl-4-methoxyphenol: 6 mL anisaldehyde was dissolved in 75 mL dichloromethane (DCM). A mixture of 12 g hydrogen peroxide and 10 mL conc. formic acid was added over 30 min. The reaction mixture was gently refluxed for 21 h.
C: B 4-methoxyphenol: Evaporating the solvent from reaction mixture and taking up the residue in 100 mL aqueous NaOH (20%) (25 mL MeOH as co-solvent) yielded 4.1 g 4-methoxyphenol as a white crystalline product after the usual work-up and purification steps.
D : Reimer-Tiemann formylation of 4-methoxyphenol: 124.1 g 4-methoxyphenol was dissolved in NaOH solution (320 g NaOH in 400 mL water). In total, 161 mL chloroform was added. The usual work-up and steam distillation yielded 109.8 g of a clear yellow oil that did not solidify upon standing at room temperature (GC/MS: 94% 2-hydroxy-5-methoxybenzaldehyde).
E: D Methylation of 2-hydroxy-5-methoxybenzaldehyde: The yellow oil from was used without further purification. A 250 mL RB flask was charged with 100 mL acetone, 14 g anhydrous potassium carbonate and 10 g 2-hydroxy-5-methoxybenzaldehyde; the mixture was brought at reflux temperature and 11 g dimethyl sulfate was added. The reaction was continued for 4 hours. The solvent is evaporated and the crude end product crystallized in cold water. Recrystallization from EtOH/water yielded 8.3 g 2,5-dimethoxybenzaldehyde (GC/MS: 98%+ 2,5-dimethoxybenzaldehyde).
You may like
Lastest Price from 2,5-Dimethoxybenzaldehyde manufacturers

US $0.00-0.00/kg2025-11-20
- CAS:
- 93-02-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons
US $1.00/KG2025-09-12
- CAS:
- 93-02-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG