Vardenafil Hydrochloride: Pharmacodynamics and Methods of Preparation
General Description
Vardenafil hydrochloride, a potent and selective PDE-5 inhibitor, is crucial in treating erectile dysfunction (ED) in men. By inhibiting PDE-5 found in the penile corpus cavernosum, Vardenafil hydrochloride boosts cGMP levels, leading to smooth muscle relaxation and enhanced blood flow, thus facilitating erection. Vardenafil hydrochloride's unique design for ED treatment makes Vardenafil significantly more effective than other PDE-5 inhibitors like sildenafil, with a higher potency that allows for lower effective doses. The recommended starting dose is 10 mg, taken about 60 minutes before sexual activity, demonstrating both efficacy and tolerability. Side effects are generally mild and include headache, flushing, dyspepsia, and rhinitis. The synthesis of Vardenafil hydrochloride involves complex chemical reactions, starting from alkylation of 2-hydrozybenzonitrile to multiple steps that yield the final product. Improved methods and advanced synthetic routes have been developed for large-scale production and research purposes, including [14C]-labeled and [3H]-labeled variants, highlighting its pharmaceutical importance and the innovation in its creation.

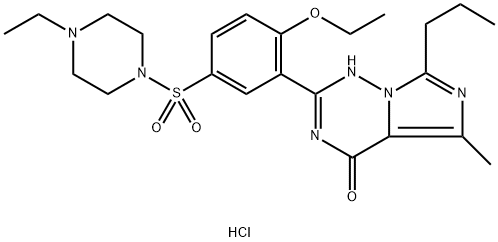
Figure 1. Vardenafil hydrochloride
Pharmacodynamics
Vardenafil hydrochloride, a highly potent and selective inhibitor of phosphodiesterase-5 (PDE-5), plays a significant role in the treatment of erectile dysfunction in men. PDE-5 is the predominant enzyme found in the penile corpus cavernosum, and its inhibition by Vardenafil leads to increased levels of cyclic guanosine monophosphate (cGMP) within the cavernous tissue. This elevation in cGMP promotes relaxation of the penile smooth muscle and increases blood flow, facilitating erection. Distinguished from other PDE-5 inhibitors like sildenafil and tadalafil, Vardenafil was specifically designed as an erectogenic agent, making it uniquely effective in this capacity. It is considered 5-10 times more potent than sildenafil, indicating its strong efficacy in lower doses. The recommended starting dose for treating ED is 10 mg, taken on demand about 60 minutes before sexual activity, which has been shown to be effective and well-tolerated. Despite its potency, the adverse effects associated with Vardenafil hydrochloride are generally mild, dose-dependent, and tend to decrease over time. Common side effects include headache, flushing, dyspepsia, and rhinitis. This profile underscores Vardenafil's safety and tolerability, making it a valuable option for men experiencing ED. 1
Methods of Preparation
The preparation of Vardenafil hydrochloride involves a series of complex chemical reactions, beginning with the alkylation of 2-hydrozybenzonitrile and proceeding through multiple steps to achieve the final product. Initially, 2-hydrozybenzonitrile is alkylated with ethyl bromide, followed by the addition of ammonia to introduce the nitrile functionality, leading to the formation of amidine. This process involves generating AlMeClNH2 in situ by combining ammonium chloride with trimethyl-aluminum. Subsequently, D,L-alanine undergoes acylation with butyryl chloride and then reacts via a Dakin–West reaction to produce an intermediate a-oxoamino ester. This ester, in turn, reacts with carboximidohydrazide, formed in situ from amidine and hydrazine hydrate, under heating to yield a condensation product. This intermediate is then cyclized to form imidazotriazinone using phosphorous oxychloride. Following this, the compound undergoes a reaction with chlorosulfonic acid to introduce a sulfonyl chloride group, which is then treated with N-ethylpiperazine to obtain Vardenafil. The dihydrochloride salt of Vardenafil is finally produced by reacting the compound with hydrochloric acid in ether. An improved method for Vardenafil dihydrochloride synthesis on a larger scale modifies the initial steps to accommodate industrial production constraints, such as avoiding the use of triethyl-aluminum. This involves dehydrating 2-ethoxybenzamide with thionyl chloride, followed by a series of reactions leading to the synthesis of benzamidine, which is then cyclized and sulfonated to ultimately yield Vardenafil dihydrochloride. Furthermore, advanced synthetic routes have been developed, including the preparation of [14C]-labeled and [3H]-labeled Vardenafil hydrochloride for research purposes. These methods involve intricate steps of synthesis, starting from labeled precursors and following through reactions that meticulously construct the Vardenafil molecule, emphasizing the compound's significance in medical research and pharmaceutical applications. 2
Reference
1. Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature, Eur. J. Med. Res. 2002;7:435-446.
2. Ashour AE, Rahman AF, Kassem MG. Vardenafil dihydrochloride. Profiles Drug Subst Excip Relat Methodol. 2014;39:515-544.
Related articles And Qustion
Lastest Price from Vardenafil hydrochloride manufacturers

US $15.00-1.00/kg2025-10-31
- CAS:
- 224785-91-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $0.00-0.00/kg2025-09-17
- CAS:
- 224785-91-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1T