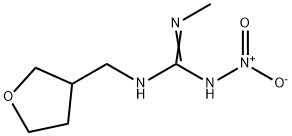
Uses of Dinotefuran
Dinotefuran is an insecticide of the neonicotinoid class developed by Mitsui Chemicals for control of insect pests such as aphids, whiteflies, thrips, leafhoppers, leafminers, sawflies, mole cricket, beetles, mealybugs, and cockroaches on leafy vegetables, in residential and commercial buildings, and for professional turf management.

Characteristics
Technical dinotefuran is an odorless white crystalline solid. It has a solubility of 39.83 g/L in water, and is highly soluble in dichloromethane, acetone, methanol, and ethyl acetate. Technical dinotefuran has a melting point of 107.5℃, and a log Pow of -0.549 at 25℃. It is nonflammable, is not explosive to thermal, shock and frictional tests, and has a vapor pressure of <1.7 x 10-6 Pa at 25℃.
Uses
Dinotefuran is a broad-spectrum insecticide, which is proposed for food uses in/on leafy vegetables (except Brassica) (group 4), and for use in professional turf management, professional ornamental production, and in the residential indoor, pet, lawn and garden markets. Dinotefuran is a neonicotinoid in the nitroguanidine sub-class, same as another insecticide clothianidin. Available product chemistry, toxicology, ecological effects and environmental fate data supporting the proposed uses have been reviewed. The data and estimated risks to human health and the environment from its proposed uses are summarized below.
Mechanism of action
Dinotefuran's mechanism of action involves disruption of the insect's nervous system by inhibiting nicotinic acetylcholine receptors. In order to avoid harming beneficial insects such as bees, it should not be applied during bloom.
Toxicokinetics
The results of the absorption and excretion studies demonstrate that 14C-dinotefuran is well absorbed from the G.I. tract of male and female adult and neonate rats into the systemic circulation and is rapidly excreted mainly in the urine. Because extensive absorption has been demonstrated, an oral absorption value of 100 % will be used in the risk assessment. There are no significant differences in absorption and excretion in adult rats after single or repeated exposure and between high and low doses of dinotefuran. There is very limited enterohepatic re-circulation of dinotefuran as indicated by the low levels of radiolabel detected in the bile.
The tissue distribution studies on 14C-dinotefuran demonstrate that following absorption from the G.I. tract the radiolabel is widely distributed in male and female adult and neonatal rats. In addition the distribution of dinotefuran and/or its metabolites extends to the foetus and to the milk of lactating rats. There is no evidence of bioaccumulation. The metabolite profiling studies demonstrate that only limited metabolism of dinotefuran occurs in vivo as <10 % of radiolabel is associated with metabolites. In addition similar metabolism pathways exist in adult males and females regardless of dosing regimen and in neonates.
You may like
Related articles And Qustion
Lastest Price from Dinotefuran manufacturers

US $0.00/kg2025-09-28
- CAS:
- 165252-70-0
- Min. Order:
- 1kg
- Purity:
- 95
- Supply Ability:
- 200000

US $0.00-0.00/KG2025-05-09
- CAS:
- 165252-70-0
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS