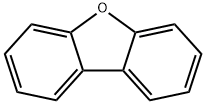
Uses of Dibenzofuran
Dioxins and furans, two well-known environmental pollutants, are extremely toxic to humans and many other species. Dibenzofurans are released into the air from combustion sources, and are listed as pollutants of concern due to its persistence in the environment, potential to bioaccumulate, and toxicity to humans and the environment. These compounds, when adsorbed on soils or other substrates, are highly persistent under normal environmental conditions. The Office of Environmental Health Hazard Assessment reviews risk assessments submitted under the Air Toxics ‘Hot Spots’ Program (AB 2588) in which chlorinated dibenzo-p-dioxins and dibenzofurans are listed. Chlorinated dibenzofurans were calculated as total 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) equivalents in the AB 2588 risk assessments.

Property
Dibenzofuran is a white crystalline solid. It is not soluble in water, but soluble in organic solvents like alcohol, acetone, ether, benzene, and acetic acid. Its boiling point is 287°C, melting point is 86°C, and specific gravity is 1.0886. Its octanol/ water partition coefficient is 4.12.
Uses
Dibenzofuran is an industrial chemical or by-product, and is used as an insecticide, in the production of PVC, industrial bleaching and incineration.
Environmental Fate
Dibenzofuran is cited in the Clean Air Act 1990 Amendments – Hazardous Air Pollutants as a volatile hazardous air pollutant of potential concern. The Superfund Amendment Reauthorization Act Section 110 placed dibenzofuran on the revised Agency for Toxic Substances and Disease Registry priority list of hazardous substances to be the subject of a toxicological profile. Dibenzofuran is also listed in the Massachusetts Substance List for Right to Know Law, the New Jersey Department of Health Hazard Right to Know Program Hazardous Substance List, the Pennsylvania Department of Labor and Industry Hazardous Substance List, and the California’s Air Toxics ‘Hot Spots’ List (Assembly Bill 2588).
Presence of dibenzofuran in coal tar, oil and its uses may result in its release to the environment through various waste streams. It has very low to no mobility in soil and degrades in soil. It dissolves in water as well as volatilizes. As a gas phase in the atmosphere, it reacts with photochemically produced hydroxy radicals. It is biodegraded rapidly at contaminated sites by the microorganisms present; otherwise its biodegradation is relatively slow. It adsorbs very strongly to sediment and particulate matter in the water column. It has a potential to bioconcentrate in aquatic organisms.
Mechanism of Toxicity
Dibenzofuran induces microsomal enzymes such as cytochrome
P450 1A1, 1A2, and aryl hydrocarbon hydroxylase in
rat liver, lung, and skin. Thus, toxicity and carcinogenicity may
result from its bioactivation through aryl hydrocarbon receptor
signal transduction pathway.
You may like
Related articles And Qustion
Lastest Price from Dibenzofuran manufacturers

US $10.00/KG2025-04-21
- CAS:
- 132-64-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-04-15
- CAS:
- 132-64-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
![53-70-3 Dibenz[a,h]anthraceneEnvironmental FateToxicity](/NewsImg/2022-01-26/6377880661226622929690346.jpg)
![53-70-3 Dibenz[a,h]anthraceneEnvironmental FateToxicity](/NewsImg/2022-01-18/6377809726988491459797031.jpg)