Uses of Bis (2-methoxyethyl) ether
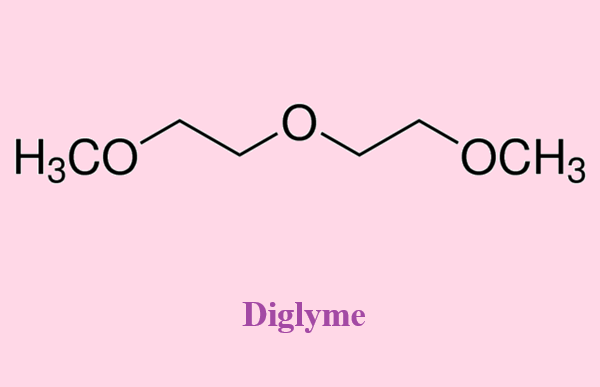
Bis (2-methoxyethyl) ether, also known as diglyme, is a linear aliphatic diether widely used as a solvent and present as a clear liquid at room temperature with a mild ether odor. The compound is notknown to occur in nature. It is synthesized from ethylene oxide and methanol in the presence of either acidic or basic catalysts. The reaction is based on the classic Williamson ether synthesis. It can also be produced from diethylene glycol and dimethyl sulfate. In June 2012, ECHA proposed addition of diglyme to the REACH very high concern list.

Uses
Bis (2-methoxyethyl) ether, due to being chemically inert and possessing excellent solvent properties, is mainly used as a solvent and an anhydrous reaction medium for organometallic synthesis. It is also used as a solubilizer.
Environmental Fate
Bis (2-methoxyethyl) ether will present as
a vapor, when released to air, at a vapor pressure of
2.96 mmHg at 25°C. The vapor phase is easily degraded in
the atmosphere by reaction with photochemically produced
hydroxyl radicals. The half-life for the reaction is estimated
to be 22 h. Bis (2-methoxyethyl) ether does not contain
chromophores that will absorb at wavelengths >290 nm
and therefore is not expected to be susceptible to direct
photolysis by sunlight.
Bis (2-methoxyethyl) ether has very high
mobility in soil based on the estimated Koc of 15, when released to soil. It may volatilize from dry soil surfaces
based upon its vapor pressure.
Aquatic fate: Bis (2-methoxyethyl) ether does not absorb to
suspended solids and sediments when released into water.
Hydrolysis is not an important environmental matter
because bis (2-methoxyethyl) ether does not contain
a functional group that can hydrolyze under environmental
conditions.
Mechanism of Toxicity
The metabolite 2-methoxyacetic acid, which is generated from 2-methroxyethanol by the reaction of alcohol dehydrogenase, may be important for the toxic effects. It can undergo activation to methoxyacetyl coenzyme A and enter the Krebs cycle or fatty acid biosynthesis. Several metabolites of 2-methoxyethanol, such as 2-methoxy-N-acetyl glycine, have been identified that support this pathway. Thus, 2-methoxyacetic acid may interfere with essential metabolic pathways of the cell, and it was hypothesized that this causes the testicular lesions and malformations in experimental animals.
You may like
Related articles And Qustion
Lastest Price from Diglyme manufacturers

US $0.00/kg2025-06-09
- CAS:
- 111-96-6
- Min. Order:
- 170kg
- Purity:
- 99%
- Supply Ability:
- 20 MT

US $10.00/KG2025-04-21
- CAS:
- 111-96-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt