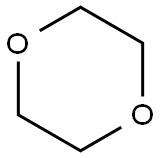
Uses of 1,4-Dioxane
1,4-Dioxane was first identified in 1863 and became available for commercial use in the 1930s as a solvent in cellulose acetate and plastics manufacturing. 1,4-Dioxane was used as a stabilizer for chlorinated organic solvents, beginning in the 1950s. Groundwater contamination has been an issue of concern near hazardous waste sites contaminated with chlorinated solvents, due to the high degree of mobility of 1,4-dioxane in soil and water systems. 1,4-Dioxane is a contaminant of ethoxylated surfactants, leading to additional concerns about the concentration of this chemical in detergents, shampoos, and cosmetics. There are many current industrial uses of this chemical as a solvent or chemical intermediate, resulting in exposure to occupational workers.
Uses
1,4-Dioxane is used as a solvent and solvent stabilizer, degreaser, wetting and dispersing agent, liquid scintillation medium, polymerization catalyst, in drug purification, and to extract animal and vegetable oils. It is used as a solvent for cellulose acetate, nitrocellulose, and other cellulose esters or ethers; fats, oils, waxes, and mineral oil; natural and synthetic resins; and polyvinyl polymers.
1,4-Dioxane is an ingredient or chemical intermediate in the manufacture of cleaning and detergent preparations, adhesives, deodorant fumigants, plastics, rubber, insecticides, herbicides, polishing compositions, lacquers, paints, varnishes, paint and varnish removers, printing compositions, and dye baths. It has been used in the embedding process for the preparation of tissue sections for histology. In addition, 1,4-dioxane is a contaminant of ethoxylated surfactants such as polyethylene glycol (PEG) that are used in personal care products and cosmetics. Vacuum stripping processes intended to remove 1,4-dioxane are generally applied prior to formulation of surfactants for use in these products. In the past, a major use for 1,4-dioxane was for the stabilization of chlorinated solvents, primarily 1,1,1-trichloroethane (1,1,1-TCA). However, production of 1,1,1-TCA was restricted in 1995 under the Montreal Protocol on Substances that Deplete the Ozone Layer; therefore, current use of 1,4-dioxane for this purpose is expected to be low.
Mechanism of Toxicity
Eye and respiratory irritation occurs from direct contact of 1,4-dioxane with mucous membranes. Pharmacokinetic and toxicological data indicate that liver and kidney toxicity induced by 1,4-dioxane occurs only after doses large enough to saturate processes for detoxification and elimination. 1,4-Dioxane is one of many carcinogens that have not been demonstrated to react significantly with DNA. Its cancer mode of action is not sufficiently well understood to permit assignment to a specific class of epigenetic agents. However, the data suggest a tumor promotion mechanism associated with tissue injury and subsequent regeneration.
Environmental Fate
1,4-Dioxane is a colorless liquid that is miscible with water and moderately volatile (vapor pressure of 38.1 mmHg at 25 ℃). In the atmosphere, 1,4-dioxane will exist in the vapor phase, and will largely be degraded via photooxidation reaction with hydroxyl radicals; the half-life for this reaction is estimated to be 35 h. 1,4-Dioxane has a low log KOW (-0.27) and estimated KOC (29). After release into the soil, it will exhibit high mobility and little adsorption, leaching readily into deeper soil and groundwater. Based on its vapor pressure, it is expected that volatilization from dry soil surfaces may also occur. If released to water, 1,4-dioxane is expected to volatilize from the surface based on its Henry’s law constant of 4.8×10-6 atm-m3 mol-1. As with soil, little or no adsorption to sediment or solids in natural water systems is expected. 1,4-Dioxane is resistant to hydrolysis and microbial degradation, and photolysis at water and soil surfaces is unlikely to be significant. Oxidation of 1,4- dioxane by aqueous hydroxyl radicals has been observed; the half-life for this reaction was 336 days. Based on its KOW, this chemical is not expected to bioaccumulate in the food chain, and available bioconcentration factors are low (in the range of 0.2–0.7).
You may like
Related articles And Qustion
See also
Lastest Price from 1,4-Dioxane manufacturers

US $100.00/KG2025-04-21
- CAS:
- 123-91-1
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON
US $0.00-0.00/kg2025-04-21
- CAS:
- 123-91-1
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons