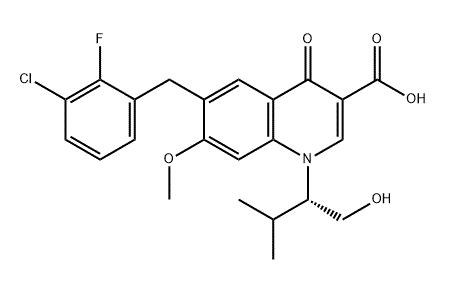
Uses and Side effects of Elvitegravir
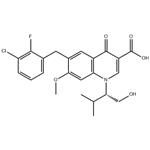
Elvitegravir (brand name Vitekta) is a drug used as part of antiretroviral therapy (ART). The FDA approved elvitegravir in 2012 as an antiretroviral drug (ARV) for people with HIV infection. Elvitegravir is manufactured by Gilead Sciences.
Elvitegravir was sold from 2014 to 2016 as a single drug known as Vitekta. Vitekta was withdrawn from the market by Gilead Sciences in 2017, although elvitegravir is still available as a component of several combination HIV medicines (see below).
Medical uses
In the United States, elvitegravir can be obtained either as part of the combination pills Stribild or Genvoya, or as the single pill formulation Vitekta.
Vitekta is FDA approved to be used for the treatment of HIV-1 infection in adults who have previous treatment experience with antiretroviral therapy. It must be used in combination with a protease inhibitor that is coadministered with ritonavir as well as additional antiretroviral drug(s).
Pharmacokinetics
Pharmacokinetics of elvitegravir are enhanced when co-administered with inhibitors of CYP3A4. Studies demonstrated ritonavir 100 mg and cobicistat 150 mg similarly enhanced elvitegravir concentrations.88 Boosted elvitegravir reaches peak serum concentrations approximately 4 hours after oral administration. Food increases systemic exposure, and elvitegravir should be taken with food. Elvitegravir is primarily metabolized by CYP3A enzymes in addition to undergoing glucuronidation via UGT1A1/3 enzymes. Elvitegravir has a terminal half-life of 13 hours when co-administered with cobicistat; elimination is primarily in feces.
Side effects
When you start any ARV, you may have temporary side effects such as headaches, nausea, indigestion, or a general sense of feeling ill. These side effects usually get better or disappear over time.
The most common side effects of elvitegravir are diarrhea, nausea, and headaches.
Elvitegravir can cause serious side effects including:
Immune Reconstitution Inflammatory Syndrome (IRIS). IRIS is a side effect that can happen when you start taking HIV medications. Your immune system might get stronger and begin to fight infections that have been hidden in your body for a long time. This may result in an inflammatory response which may require further evaluation and treatment. Tell your healthcare provider right away if you experience any new symptoms after starting elvitegravir.
These are not all the possible side effects of elvitegravir. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may like
Lastest Price from Elvitegravir manufacturers
US $0.00/kg2025-11-25
- CAS:
- 697761-98-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $15.00-10.00/KG2021-07-13
- CAS:
- 697761-98-1
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton