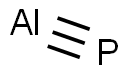Use of Aluminum phosphide
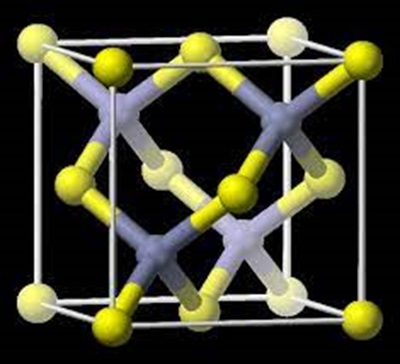
Aluminium phosphide is a highly toxic inorganic compound with the chemical formula AlP used as a wide band gap semiconductor and a fumigant. This colorless solid is generally sold as a grey-green-yellow powder due to the presence of impurities arising from hydrolysis and oxidation.

Use
The primary use for Aluminum phosphide is as a fumigant to control insects and rodents in both food and nonfood crops in indoor environments. It is also used in the control of rodents outdoors via application to their burrows or in grain storage areas. Due to the extremelycommonuse of this compound for protecting rice, it is also called rice tablet in some countries. Aluminum phosphide is formulated in solid form and is available for use as a tablet, pellet, or dust. It is marketed as dark gray 3-g tablets consisting of Aluminum phosphide (56%) and carbamate (44%), under brand names such as Celphos, Alphos, Quickphos, Phosfume, Phostoxin, Talunex, Degesch, Synfume, Chemfume, Phostek, and Delicia in porous hags or blister packs.
Environmental Fate
Once exposed to water or in the presence of high ambient humidity, Aluminum phosphide generates phosphine gas. Therefore, atmospheric dissipation is expected to be the primary fate process for phosphine. In addition to phosphine being generated from the reaction of Aluminum phosphide with water, the other reaction product is aluminum hydroxide, a common constituent of clay. If the liberated phosphine (PH3) burns, it will produce phosphorus pentoxide (P2O5), which forms orthophosphoric acid (H3PO4) when exposed to water.
Mechanism of action
Phosphine is known to bind to and inhibit cytochrome oxidase and changes the valence of the hem component of hemoglobin. Oxidative stress is one of the main mechanisms of action of Aluminum phosphide toxicity, which boosts extramitochondrial release of free oxygen radicals resulting in lipid peroxidation and protein denaturation of the cell membrane in various organs. Furthermore, Aluminum phosphide reduces glutathione, which is one of the main antioxidant defenses.
Aluminum phosphide causes toxic stress, accompanied by changes in glucose metabolism. It also disrupts protein synthesis and enzymatic activity, particularly in the lung and heart cell mitochondria, which leads to blockage of the mitochondrial electron transport chain. Phosphine may cause denaturing of various enzymes; it is involved in cellular respiration and metabolism, and may be responsible for denaturation of the oxyhemoglobin molecule.
You may like
Related articles And Qustion
Lastest Price from Aluminum phosphide manufacturers

US $1.00/g2022-12-29
- CAS:
- 20859-73-8
- Min. Order:
- 10g
- Purity:
- 99.5%
- Supply Ability:
- 1000kg

US $50.00/KG2021-08-31
- CAS:
- 20859-73-8
- Min. Order:
- 1Kg/Bag
- Purity:
- 99.99%
- Supply Ability:
- 20 tons/month