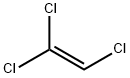
Trichloroethylene: Properties, Production process and Uses
Trichloroethylene, also known as chlorylene, 1,1,2-trichloroethylene, 1,1,2-trichloroethene,
acetylene trichloride, 1-chloro-2,2-dichloroethylene, 1,1-dichloro-2-chloroethylene, trichlorethene and 1,2,2-trichloroethylene, is a derivative of acetylene and chlorine. Molecular formula: C2HCl3, molecular weight: 131.388. The chemical
structure is:
Properties of trichloroethylene
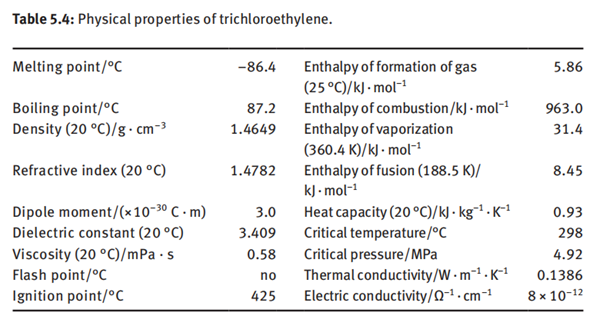
Trichloroethylene is a colorless and transparent liquid with an odor similar to chloroform. It is volatile and nonflammable. Trichloroethylene is insoluble in water. It is soluble in most organic solvents such as ethanol and ether. Trichloroethylene forms an azeotrope with water, having an azeotropic point of 73.6°C and azeotropic composition: trichloroethylene of 94.6% and water of 5.4%. Trichloroethylene has an anesthetic effect. It can cause anesthesia after 20 min in the air containing 400 × 10−6of trichloroethylene. It will lose consciousness after 10 min in the air containing 3,000 × 10−6 of trichloroethylene. The physical properties of trichloroethylene are shown in Table 5.4.
Usually, a stabilizer is added to the trichloroethylene during storage to prevent it from auto-oxidation. The trichloroethylene containing no stabilizer is gradually oxidized in the air to form an intermediate dichloroacetyl chloride and subsequently generate phosgene, carbon monoxide and hydrogen chloride:
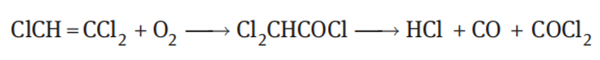
The reaction proceeds according to the radical mechanism, and light and heat significantly accelerate the reaction. In the presence of water, dichloroacetyl chloride is decomposed into dichloroacetic acid and hydrogen chloride. The acid produced by the decomposition corrodes the metal. Therefore, a trace amount of a stabilizer such as hydroquinone or an amine is added to it. In the presence of air, moisture and light, even if it is heated to 130°C, the trichloroethylene with addition of a stabilizer has no effect on metal materials generally used in industry. When trichloroethylene vapor is heated to more than 700°C, it decomposes into a mixture of dichloroethylene, tetrachloroethylene, carbon tetrachloride and chloroform.
Trichloroethylene is not easily hydrolyzed under normal conditions of use, but
it can be hydrolyzed to chloroacetic acid by heating to 90°C in the presence of 90%
sulfuric acid:
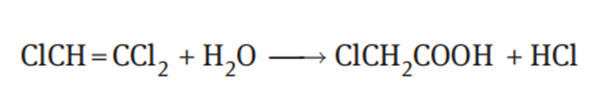
In the presence of a copper salt, trichloroethylene is heated to 175°C under pressure and reacts with an aqueous solution or suspension of an alkali metal or alkaline earth metal hydroxide to form a hydroxyethyl salt. Trichloroethylene does not react with hydrochloric acid or nitric acid at low temperature. However, upon heating, trichloroethylene reacts violently with concentrated nitric acid and decomposes completely into trichloronitromethane or monochlorodinitromethane according to the reaction conditions. In the presence of aluminum trichloride, trichloroethylene reacts with hydrogen chloride to form 1,1,1,2-tetrachloroethane, with chloroform to form 1,1,1,2,3,3-hexachloropropane, and with carbon tetrachloride to form 1,1,1,2,3,3,3- heptachloropropane.
Aluminum, especially aluminum powders can cause the decomposition of the
trichloroethylene without a stabilizer to generate hydrogen chloride, and at the same
time, a strong explosion occurs.
Production process of trichloroethylene
The industrial processes for production of trichloroethylene include three routes: chlorination of acetylene, direct chlorination of ethylene, and oxychlorination of ethylene. Before the 1970s, the production of trichloroethylene by chlorination of acetylene dominated, but since the 1980s, due to the rapid development of petrochemical industry, ethylene direct chlorination and ethylene oxychlorination have gradually become mainstream, but in China, trichloroethylene is mainly produced by chlorination of acetylene.
Chlorination of acetylene
Acetylene reacts with chlorine to form tetrachloroethane, and then the resultant tetrachloroethane is saponified to produce trichloroethylene. The reaction equations are as follows:
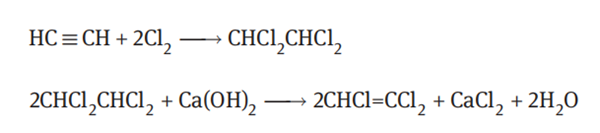
Acetylene and chlorine are introduced into a chlorination reaction tower containing a
tetrachloroethane solvent and ferric chloride or antimony trichloride catalyst to start
the reaction at a temperature of 70–90°C and under a pressure of 9.8–29.4 kPa. The
molar ratio of chlorine to acetylene is 2:2.05, and the dosage of ferric chloride catalyst is 0.1%. The heat of reaction is removed by the circulation of tetrachloroethane
to an external heat exchanger. The obtained tetrachloroethane is introduced into
an intermediate storage tank and sent to the trichloroethylene reaction tower for a
saponification reaction with 10% lime milk at a temperature between 70 and 100°C.
Hydrogen chloride is removed from tetrachloroethane to give trichloroethylene. Steam
is introduced into the bottom of the tower to boil the reaction solution. The azeotrope
of trichloroethylene and water is distilled off from the top of the tower, condensed and
separated into two layers. The organic layer is collected and subjected to fractional distillation towers for removal of the light and heavy components. The finished product of
trichloroethylene is obtained with a total yield of more than 95%. Production of 1 ton of
trichloroethylene consumes 198 m3
of acetylene, 1,350 kg of chlorine and 2.4 t of steam.
Direct ethylene chlorination
Ethylene is directly chlorinated to obtain a mixture of tetrachloroethane and pentachloroethane, which are subjected to gas phase cracking to obtain trichloroethylene, and by-product tetrachloroethylene. The reaction equations are as follows:
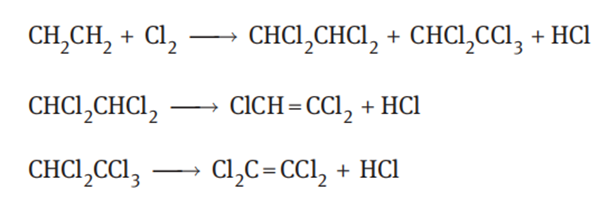
Ethylene and chlorine were introduced into the reaction tower, and the ratio of the raw
materials could be adjusted according to the desired product ratio. The conversion of
ethylene was 95% to 98%. The reaction solution was sent to a deacidification tower to
remove dissolved hydrogen chloride. Subsequently, the solution was introduced into
a fractional distillation tower. The top product was sent back to the reaction tower.
The bottom product (a mixture of tetrachloroethane and pentachloroethane) was
introduced into a cracking furnace for splitting into trichloroethylene and tetrachloroethylene at a temperature of 400 to 500°C in the presence of a barium chloride
catalyst supported on activated carbon. The outlet gas from the cracking furnace was
quenched and separated from hydrogen chloride. Then, the resulting solution was
introduced into fractional distillation towers for removal of light and heavy components, and finally refined trichloroethylene and tetrachloroethylene were obtained.
In this process, the production of trichloroethylene is always accompanied by the by-product tetrachloroethylene. The ratio of the two products can be adjusted by changing the ratio of raw materials.
Oxychlorination of ethylene
Ethylene undergoes an oxychlorination with chlorine and oxygen or air to obtain a
trichloroethylene and tetrachloroethylene:
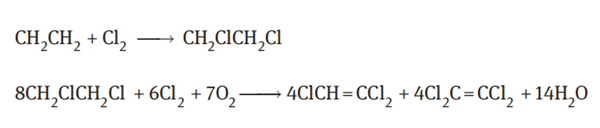
Using copper chloride or potassium chloride as a catalyst, 85–90% of ethylene was converted into chlorinated hydrocarbons, and 10–15% was converted into carbon monoxide and carbon dioxide in a bubbling bed reactor at a temperature of 425°C. At a higher reaction temperature, the side reaction such as decomposition was significant; and at lower reaction temperature, the generation of tetrachloroethane byproduct was increased.
Uses of trichloroethylene
Trichloroethylene is an excellent solvent that can be used as a substitute for benzene
and gasoline. It can be used to dissolve resins, asphalt, coal tar, cellulose acetate,
nitrocellulose, rubber and coatings. Trichloroethylene is applied as a dry cleaning
agent for textiles and wool, a surface treatment agent for metals, a cleaning agent
for electronic components. It is also used as a raw material for production of tetrachloroethylene. In recent years, trichloroethylene has been used in large quantities
to produce dichloroacetyl chloride, octachlorodipropyl ether, new refrigerants HFC-
134a, HFC-123 and HFC-125. In addition, it is also used as caprolactam, pesticide
extractant, general anesthetic, monochloroacetic acid and other organic synthesis
intermediates.
Lastest Price from Trichloroethylene manufacturers

US $0.00/kg2025-06-14
- CAS:
- 79-01-6
- Min. Order:
- 300kg
- Purity:
- 99%
- Supply Ability:
- 20 MT

US $1.00/KG2025-03-07
- CAS:
- 79-01-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt