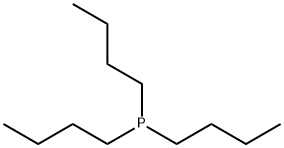
Tributylphosphine: Applications in Organic Synthesis and its Health Hazards
Description
Tributylphosphine(TBP) is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with other oxidizing agents, to give the corresponding phosphine oxide. It is usually handled using air-faree techniques.
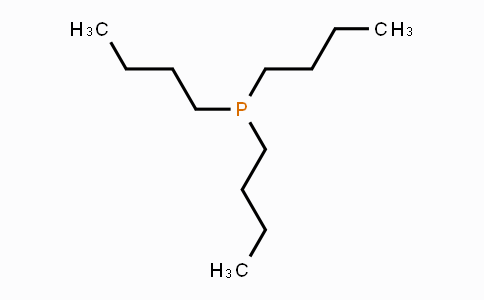
Reactions
Tributylphosphine (TBP) is a powerful reductant that need to be present in only slight molar excess for the complete reduction of disulfides; moreover, it do not react with thiol-specific reagents and do not give rise to gas formation during the reaction.
Applications in Organic Synthesis
Tributylphosphine finds some industrial use as a catalyst modifier in the cobalt-catalyzed hydroformylation of alkenes, where it greatly increases the ratio of straight-chain aldehydes to branched-chain aldehydes in the product mixture.
It is the precursor to the pesticide (2,4-dichlorobenzyl)tributylphosphonium chloride ("Phosfleur").1
Tributylphosphine is a common ligand for the preparation of complexes of transition metals in low oxidation states. It is cheaper and less air-sensitive than trimethylphosphine and other trialkylphosphines.
Health Hazards
Tributylphosphine is moderately toxic, with an LD50 of 750 mg/kg (oral, rats).
References:
[1] SVARA J, WEFERLING N, HOFMANN T. Phosphorus Compounds, Organic[C]. 2006. DOI:10.1002/14356007.A19\_545.PUB2.You may like
See also
Lastest Price from Tributylphosphine manufacturers

US $10.00/ASSAYS2025-08-21
- CAS:
- 998-40-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $30.00-10.00/KG2025-04-15
- CAS:
- 998-40-3
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg