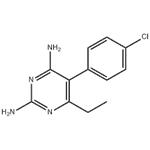
Toxicity of Pyrimethamine
Pyrimethamine, sold under the brand name Daraprim among others, is a medication used with leucovorin (leucovorin is used to decrease side effects of pyrimethamine; it does not have intrinsic anti-parasitic activity) to treat the parasitic diseases toxoplasmosis and cystoisosporiasis.It is also used with dapsone as a second-line option to prevent Pneumocystis jiroveci pneumonia in people with HIV/AIDS.
Toxicity
Toxicity associated with pyrimethamine alone and pyrimethamine in combination with dapsone (maloprim) is discussed below, while toxicity associated with the combination of pyrimethamine+sulfadoxine (Fansidar) is discussed elsewhere.
Neurotoxicity
High dosages of pyrimethamine have been associated with a variety of nervous system side-effects, including ataxia, tremors, and seizures, and occasionally with less specific symptoms such as insomnia, depression, fatigue, malaise, headache, lightheadedness, and irritability. Marked mental obtundation has been associated with hyperammonemia and carnitine deficiency induced by treatment with pyrimethamine and sulfadiazin. 6f.
Eosinophilia and respiratory toxicity
Marked eosinophilia, including pulmonary eosinophilia, has been associated with administration of maloprim; however, this is generally considered to be due to the dapsone component inducing a ''dapsone syndrome''. Nevertheless, reports of pulmonary eosinophilia in patients taking Fansidar or pyrimethamine+chloroquine have raised the possibility that pyrimethamine is responsible for this adverse reaction. 6g.
Nephrotoxicity
Increases in serum creatinine have been noted in some patients treated with pyrimethamine and dapsone. Opravil et al. (1993) studied six healthy volunteers after a single, combined dose of 100 mg pyrimethamine and 200 mg dapsone and noted increases in serum creatinine over a 28-hour period post dose from 81714 to 102716 mmol/l (p=0.002), with a corresponding reduction in creatinine clearance from 125727 to 91726 ml/min (po0.02), but no change in inulin clearance, blood urea nitrogen, or b2-microglobulin. All changes were reversible within 21 days, and subsequent studies of both pyrimethamine alone and dapsone alone identified pyrimethamine as the agent responsible for the reduction in creatinine clearance. Similar studies in nine HIV-infected males before and after prophylaxis for 1 month with 75 mg pyrimethamine+200 mg dapsone weekly (i.e. total of four doses) identified an analogous rise in serum creatinine from 69717 to 87732 mmol/l (po0.05), but both creatinine and inulin clearances were unchanged. Thus, pyrimethamine appears to reversibly inhibit renal tubular secretion of creatinine without affecting the glomerular filtration rate. Hematuria has been reported rarely with pyrimethamine.
Clinical use
Pyrimethamine is the mainstay, and currently the most effective therapy, for all forms of toxoplasmosis in adults and children, when it is generally used in combination with other agents such as sulfadiazine or clindamycin. The concentration of pyrimethamine necessary to inhibit or kill toxoplasma tachyzoites has not been clinically established and, importantly, pyrimethamine is inactive against toxoplasma cysts.
After ingestion of toxoplasma oocytes, the organisms rapidly transform into tachyzoites which multiply in tissue macrophages and disseminate via blood and the lymphatic system to the brain, heart, and lungs. In the immunocompetent host, the development of immunity is associated with the transformation of tachyzoites into latent cysts (bradyzoites), especially in brain and muscle, which may reactivate at times of reduced host immunity. Thus, most immunocompetent hosts do not develop significant clinical disease and generally do not require treatment.
Occasionally, primary infection is associated with chorioretinitis, meningoencephalitis, myocarditis, or pneumonitis due to unrestrained multiplication of tachyzoites, but such disease usually suggests some degree of host immunosupppression. Congenital infection may result in spontaneous abortion, fetal death, or severe disease (hydrocephalus, hepatosplenomegaly with jaundice, chorioretinitis, mental retardation). Treatment is usually with pyrimethamine and sulfadiazine, but the duration of treatment varies depending on the clinical situation.
Related articles And Qustion
Lastest Price from Pyrimethamine manufacturers
US $0.00/kg2025-11-21
- CAS:
- 58-14-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/kg2025-10-21
- CAS:
- 58-14-0
- Min. Order:
- 1kg
- Purity:
- 99%-101%; BP/USP
- Supply Ability:
- 500KGS