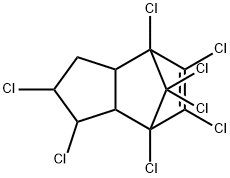
Toxicity of Chlordane
Chlordane is a chlorinated cyclodiene manufactured for use
as an insecticide. Technical chlordane is a mixture of cisand
trans-chlordane, lesser amounts of heptachlor, nonachlor
and chlordenes, and other related compounds.
Chlordane represents the oldest generation of the chloride
channel blocker insecticides with marked mammalian
toxicity.
Chlordane was first registered in the United States in
1948, and was extensively used to control agricultural and
structural pests. In the late 1970s, concerns regarding carcinogenicity
potential, toxicity to developing nervous and
immune systems, environmental persistence, and bioaccumulation
in the food chain led to its ban in many
countries by 1998.
Uses
From 1948 to 1978, chlordane was extensively used to control termites in homes by underground applications around the foundation. It was widely applied as an insecticide on agricultural crops (e.g., corn and citrus); on lawns and gardens; as a wood preservative to control borers, termites, and dry rot; for protective treatment of underground cables; and as a fumigating agent. Prior to the severe worldwide restricted use in 1980s, the estimated annual production was 9500 tons in theUnited States and 70 000 tons globally.
In 1978, the US Environmental Protection Agency (EPA) canceled its use on food crops and phased out the remaining uses. All uses and sale in the United States were prohibited in 1988, and production for export ceased in 1998. Chlordane was banned in the European Union in 1981, Canada in 1995, Australia in 1997, and Mexico in 1998. Despite these major actions to eliminate the use of chlordane, production facilities exist in China, Singapore, India, and Argentina; its insecticidal use continues in the developing countries.
Environmental Fate
Chlordane is soluble in organic solvents (e.g., cyclohexanone,
benzene) but practically insoluble in water.Chlordane does not undergo significant biotic and abiotic
degradation in soil and water. Chlordane may persist for
>20 years in soil. The degradation rate under field conditions is
4–28% per year. In river water under sunlight, 85% of chlordane
persisted to the end of the 8-week experiment. Abiotic
degradation (hydrolysis, oxidation, dechlorination, or direct
photolysis) is also not an important environmental fate
process. The estimated Log Koc of 3.5–4.6 predicts strong soil
adsorption and resistance to leaching to groundwater.
Chlordane in soil and sediment can be taken up by organisms
and plants. Due to its stability, it can travel long distances
and contaminate areas remote from its use. The measured Log
Kow of 5.4 indicates extensive bioaccumulation in food chains.
Mechanism of Toxicity
The primary target for chlordane toxicity is the central nervous system. Like other cyclodiene insecticides, chlordane acts as a noncompetitive antagonist of the chloride ion channel of the GABAA-receptor. When activated by GABA, the GABAA-receptor increases Cl-1 conductance into the neurons and prevents excessive nerve stimulation. Chlordane binds to the Cl-1channel of the receptor and thereby blocks the actions of GABA. Seizures, vomiting, and convulsions are typical symptoms associated with antagonism of GABA. The action of chlordane in the brain is very complex due to its biotransformation to more toxic metabolites and the presence of toxic components. Molecular modeling and binding studies indicate that the metabolites oxychlordane and heptachlor epoxide also block the brain GABA-gatedCl-1channels. The structurally similar component nonachlor causes toxicity symptoms typically associated with antagonism of GABA receptors.
Liver is another target of chlordane toxicity (hypertrophy, necrosis, and tumors). However, the mechanism is unknown. Chlordane induces hepatic cytochrome CYP enzymes. In mouse brain and liver, chlordane initiated signal transduction processes characteristic for known mitogens, that is, alter cellular Ca2+levels and induce protein kinase C.Otherpotential targets include adenosine triphosphatase functions, nervous system development, inflammatory cytokines, macromolecule synthesis, DNA methylation, steroid metabolism, testosterone and neurotransmitter receptors, and lipid peroxidation.