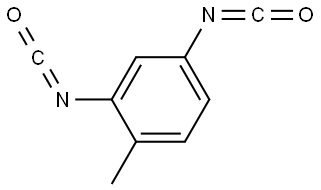
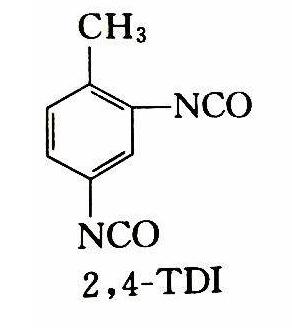
Tolylene-2,4-diisocyanate: Dermal Exposure and carcinogenicity
General Description
Toluene diisocyanate (TDI) is a highly reactive compound used to produce polyurethane foams and other industrial products, such as elastomers, coatings, and adhesives. The two most commonly used isomers of TDI are 2,4-TDI (Tolylene-2,4-diisocyanate) and 2,6-TDI (Tolylene-2,6-diisocyanate), and the main commercial TDI product is a mixture of 80% 2,4-TDI and 20% 2,6-TDI (known as 80/20 TDI).

Carcinogenicity
TDI is a liquid at room temperature, and human exposure mainly occurs through inhalation of vapours or aerosols in workplaces where TDI is produced or used. Dermal exposure to TDI is also possible via liquid TDI coming into contact with the skin during some operations, from accidental spills or splashes, or through direct handling of uncured polyurethane foam. Based on increased incidences of tumours in rodents exposed to TDI by oral gavage, TDI is classified as possibly carcinogenic to humans by the International Agency for Research on Cancer, a suspect human carcinogen by the European Union (EU), and reasonably anticipated to be a human carcinogen by the United States (US) National Toxicology Program.
The analysis of researchers indicated that the carcinogenic activity of TDI in the oral gavage bioassay was mediated by 2,4-toluene diamine (2,4-TDA), a reaction product of 2,4-TDI that is a known rodent tumorigen but is not formed in the body after inhalation exposure to TDI. By contrast, the 2,6-TDA isoform is not carcinogenic in rodents.
Dermal Exposure
ACGIH's evaluation focused on inhalation exposure, but it has been suggested that dermal exposure to TDI may contribute to TDI-associated health effects. Unfortunately, there is a dearth of quantitative data on dermal exposures. Further, those who have attempted to quantify such exposure generally report that dermal exposure to TDI varies substantially. Another complicating factor is that personal protective equipment (PPE) usage has changed over time, and the prevalence of glove use in any given study is often unknown. Still, it is important to consider that, for example, concerning foam workers, some studies suggest that the concentration of TDI in freshly made foam declines rapidly, and there was no measurable migration from intact, cured foam. Further, animal studies have shown that very little dermally applied TDI is absorbed and reaches systemic circulation (< 1%) and that dermal exposure alone is insufficient to induce respiratory sensitization in animals. This indicates that while there remains some uncertainty, the available evidence does not support that dermal exposures contribute substantially to the risk of OA in TDI workers.
Reference
1. Lynch HN, Prueitt RL, Goodman JE. Critique of the ACGIH 2016 derivation of toluene diisocyanate Threshold Limit Values. Regul Toxicol Pharmacol. 2018; 97: 189-196.
2. Prueitt RL, Lynch HN, Zu K, Shi L, Goodman JE. Dermal exposure to toluene diisocyanate and respiratory cancer risk. Environ Int. 2017; 109: 181-192.
Related articles And Qustion
Lastest Price from Tolylene-2,4-diisocyanate manufacturers

US $500.00/kg2024-11-07
- CAS:
- 584-84-9
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 200000
US $0.00/KG2023-04-28
- CAS:
- 584-84-9
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 100000