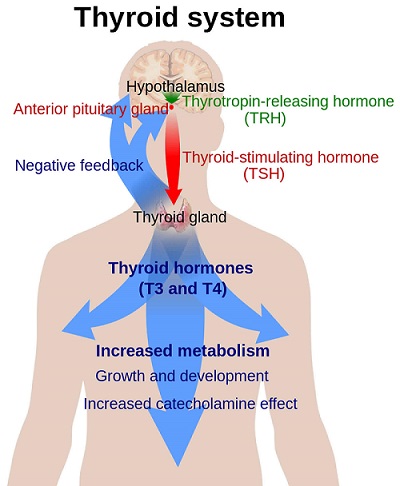
Thyrotropin(TSH)-Chemistry, Action, Preparations and Stimulation Test
Chemistry
Thyroid stimulating hormone (TSH) is a glycoprotein of MW 28,000 that is synthesized by thyrotrope cells of the anterior pituitary. It is chemically related to the family of glycoproteins, including the pituitary gonadotropins LH, FSH, and placental chorionic gonadotropin (CG). Each of these hormones contains two noncovalently linked dissimilar polypeptide chains, alpha and beta.
LH, FSH, and TSH all share similarities in tertiary structure conferred by the evolutionary preservation of cysteine residues, particularly those involved in the “cysteine knot” motif identified by crystallographic information. Furthermore, sites of N-glycosylation also appear to be preserved during evolution. As described below, glycoproteins contribute to the tertiary structure as well as the functional and immunogenic characteristics of the dimers (Zerfaoui and Ronin, 1996).
Alpha and Beta Subunits
TSH is a heterodimer composed of two noncovalently bound subunits, alpha and beta. The subunits are synthesized as separate peptides from distinct mRNAs (Vamvakopoulos and Kourides, 1979). The alpha subunit is common to all three hormones, but the beta subunit is unique for each hormone and confers biological specificity. For all four glycoproteins within each species, the amino acid sequence of the alpha subunit is common. However, the carbohydrate structure may vary. Microheterogeneity of the carbohydrate constituents of the individual hormones causes heterogeneity in receptor affinity, biological potency, and metabolic clearance (Pierce and Parson, 1981;Wondisford et al., 1988).
The alpha subunits are approximately 20 to 22 kD, have 92 to 96 amino acid residues, and contain two N-linked carbohydrate groups. The human, cow, mouse, dog, cat, and rat alpha subunit genes are similar (Yang et al., 2000b; Rayalam et al., 2006a, 2006b). All species have a single mRNA species that is between 730 and 800 bases long. The mRNA encodes the precursor of the alpha subunit and a leader sequence of an average of 24 amino acids.
The TSH beta subunit is approximately 18 kD, and consists of approximately 110–113 amino acids and contains one N-linked complex carbohydrate (Green and Baenziger, 1988). TSH, like LH, contains sulfate groups that terminate certain chains; such sulfation is found only to a small extent in FSH and not at all in CG. The genes for beta subunit of TSH of mouse, cow, human, dog, and horse have been cloned and recombinant canine TSH (Yang et al., 2000a, 2000b) and feline TSH(Rayalam et al., 2006a, 2006b) have been expressed in vitro. Each mRNA is approximately 700 bases in length with minor variations. The TSH beta mRNA encodes the precursor TSH beta subunit with a 20–amino-acid leader sequence and a 117– or 118–amino-acid coding region.
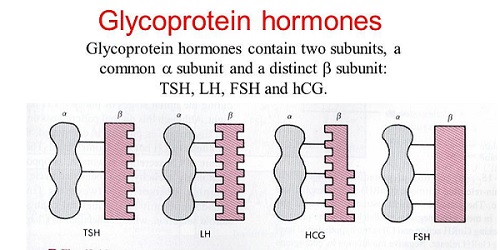
The alpha subunit peptide is more abundant than the unique beta subunit of peptides. Free serum alpha subunits are secreted and are present in equivalent concentrations to combined concentration of TSH, LH, and FSH. Free serum beta subunit concentrations are lower, usually below the level of detectability. Overabundance of alpha subunit suggests that regulation of beta subunit synthesis is the rate-limiting step in modulating the levels of TSH, LH, and FSH (McNeilly et al., 2003; Taylor and Weintraub, 1985).
Action
TSH binds to specific receptors on the thyroid plasma membrane. TSH receptors (TSHR) from dog, cat, pig, human, and rat have been completely cloned and species comparisons reveal a high degree of homology of more than 90%. The extracellular domain of the receptor contains 398 amino acids with five sites for N-linked glycosylation. The intracellular domain has 346 amino acids with seven putative transmembrane segments. The stimulatory guanine nucleotide regulatory protein binds to the third intracellular loop. The receptor has a glycoprotein component and a ganglioside, which may be involved in TSH activation of adenylate cyclase. TSHR is unique among glycoprotein hormone receptors in that some mature receptors on the cell surface are cleaved into two subunits (Rapoport et al., 1998). Only intact TSH binds to the receptor and the beta subunit does not possess biological activity (Chan et al., 1987; Field, 1975). Binding of TSH to its receptor activates adenylate cyclase and subsequent accumulation of cAMP, but is not dependent on the interaction of the TSH from that species with the receptor. This results in the stimulation and dissociation of the regulatory and catalytic subunits of cAMP-dependent protein kinase (protein kinase A) with subsequent phosphorylation of various cellular proteins resulting in an increase in thyrocyte iodide uptake, thyroid hormone organification, and thyroid hormone secretion. This cyclic AMP–mediated pathway appears to be important in both thyroid hormone secretion and thyroid glandular growth (Chayoth et al., 1985). Another important function of TSH in this context would be the up-regulation of sodium iodide symporter (NIS) in the thyroid cells. Several in vivo and in vitro studies showed that TSHstimulates not only iodide transport activity but also NIS gene and protein expression (Kogai et al., 1997, 2000; Saito et al., 1997).
The binding of TSH is also known to activate the phospholipase C signaling system. Activation of phospholipase C results in hydrolysis of PIP2 with formation of DAG and IP3. The former activates a Ca2+- phospholipid–dependent protein kinase (protein kinase C) and the latter increases intracellular Ca2+ concentrations. The effect of TSH on phospholipase C is slower and requires larger amounts of the hormone than its activation of adenylate cyclase, suggesting a high-capacity and low-affinity TSH binding site. The physiological significance of activation of PIP2 hydrolysis by TSH is not known, but is suspected to be involved in thyroid glandular proliferation (Chayoth et al., 1985; Taguchi and Field, 1988).
Preparations
Two bovine thyrotropin products have been available in the past. Availability of biochemically purified native TSH from bovine pituitary glands as a pharmaceutical has been drastically reduced following the introduction of human recombinant TSH and following the emergence of bovine spongiform encephalopathy. In addition, significantly higher endotoxin levels were detected in commercially available bTSH products, discouraging their use to prevent side effects (Schaefer et al., 2013). Nonetheless, investigators occasionally still use cell culture grade bTSHproduct for animal use. Although canine and feline recombinant TSH could successfully be synthesized, it is not commercially available (Yang et al., 2000a; Rayalam et al., 2006a). Regardless of the source, it is stored in a lyophilized form for reconstitution and parenteral administration. A study evaluated the effects of freezing reconstituted bovine TSH and demonstrated that bioactivity remained intact for at least 3 weeks at 4◦C (Bruyette et al., 1987; Ferguson, 1994). Furthermore, the effect of storage conditions on the use of recombinant human thyrotropin (rhTSH) for TSH stimulation testing in dogs indicated that reconstituted rhTSH can be stored at 4◦C for 4 weeks and at −20◦C for 8 weeks without loss of biological activity (De Roover et al., 2006).
Thyrotropin Stimulation Test
The administration of exogenous bovine or human TSH followed by the measurement of serum T4 and/or T3 is a procedure still used occasionally to confirmthe diagnosis of hypothyroidism, or loss of thyroid secretory reserve. Historically, it has been applied primarily in the dog, cat, and horse. The availability of immunoassays measuring endogenous canine TSH have reduced the practicality and need for this test. However, the TSH stimulation test continues to be considered a noninvasive test for confirming the reduced thyroid functional reserve associated with hypothyroidism.
Protocols for this test in the dog vary widely and are summarized in Table 26.8. Although the TSH dose and serum sampling times are often dictated by practical and economic considerations, increasing the TSH dose administered generally delays the time of the T4 peak, and, up to a limit, results in a higher serum T4 response and a plateau that is maintained for a longer period of time. The route of administration may be intravenous, intramuscular, or subcutaneous; however, the most consistent and rapid response is seen after intravenous dosing. For the dog, the suggested protocol is to draw a baseline blood sample for serum T4 determination, and then administer 0.1 IU/kg TSH intravenously (maximum dose 5 units), followed by a blood sample at 4 or 6 hours post-TSH (Ferguson, 1984, 1994, 2007). Studies using 1 unit of bovineTSH(bTSH) per dog have indicated that the mean increase in serum T4 and T3 above baseline at 6 hours post-TSHwas significantly lower following TSH at 1 IU than TSH at 5 IU but was not significantly different at 4 hours post-TSH for the two doses of TSH evaluated. Based on the criteria for adequate response to TSH, TSH at 1 IU led to classification of 35% of the dogs as having a decreased response to TSH at 4 hours and 35% at 6 hours. TSH at 5 IU resulted in no dogs having a decreased response at 4 hours and 1 dog in 20 (5%) at 6 hours (Beale et al., 1990).
Pituitary-source preparations of TSH have been standardized according to bioactivity, which is reported as International Units (IU). Highly purified TSH approaches a biological specific activity of 12.5 IU activity/ mg protein. A study compared the biological activity of recombinant human TSH (rhTSH) with bTSH in dogs, where bTSH was administered (1 unit corresponding to 500 μg in 0.5 ml of sterile water) intramuscularly and rhTSH was administered (75 μg in 0.5 ml sterile water) both intramuscularly and intravenously. Blood samples were collected immediately before and 6 hours after TSH administration. There were no significant differences in post-TSH stimulation T4 concentrations among the three groups (Boretti et al., 2006). Another study suggested that 50 μg rhTSH was the optimal dose in euthyroid dogs to get a significant increase in serum total thyroxine (TT4) concentrations (Sauv´e and Paradis, 2000). Similarly, IV administration of 25–200 μg rhTSH significantly increased serum TT4 concentrations 6– 8 hours later in euthyroid cats (Stegeman et al., 2003). A previous study with bovine TSH in cats had shown that the peak of serum T4 was simply extended to later time points with higher dosages (Hoenig and Ferguson, 1983). Assuming a pharmacological dosage of TSH is used, the diagnosis of hypothyroidism in the dog is usually confirmed when the post-TSH serum T4 concentration is below the normal range for basal T4 (usually <1.0 μg/dl or <13 nmol/l in the dog) and rarely increases greater than 0.2 μg/dl (2.6 nmol/l) above the baseline. In pituitary forms of hypothyroidism, the thyroid gland should remain responsive to TSH. The rare cases of longstanding secondary (pituitary) or tertiary (hypothalamic) hypothyroidism with subsequent thyroid atrophy may require two or three consecutive daily doses of TSH to eventually demonstrate thyroid responsiveness (Ferguson, 1984, 1994, 2007).
A study of normal dogs evaluated the predictive value of blood sampling times after 5 units of intravenous TSH: in 80% of the animals, a doubling of the serum T4 concentration was not achieved by 4 hours but was achieved in all cases by 6 hours post-TSH. Animals that responded favorably to thyroid replacement therapy have, on average, virtually no increase in serum T4 concentration followingTSH. An advantage of theTSHstimulation test is that post-TSHT4 concentrations tend to be less variable because the thyroid is maximally stimulated. Thyrotropin: Structural Homology Among Species The TSH beta subunit is approximately 18 kD in molecular weight, consists of approximately 110–113 amino acids, and contains one N-linked complex carbohydrate.
TSH, like LH, contains sulfate groups that terminate certain chains; such sulfation is found only to a small extent in FSH and not at all in CG. The genes for the beta subunit of TSH of mouse, rat, human, cattle, sheep, dog, cat, horse, opossum, teleost fish, chicken, quail, and horse have been cloned and sequenced. For most mammalian species, the mRNA is approximately 700 bases in length with minor variations. The TSH beta mRNA encodes the precursor TSH beta subunit with a 20-amino-acid leader sequence and a 117– or 118–amino-acid coding region. There is 80 to 90% homology at the amino-acid level among the sequences of most of the mammalian TSH beta molecules. As an example of avian species, the amino acid sequence of the quail TSH beta subunit shows homologies of about 70% to that of mammalian species, about 60% to that ofamphibian, and about 50% to that of teleost fish. There is evidence that the functional domains of the TSH beta subunit and the TSH receptor have diverged cooperatively during evolution. Many regions of identical sequences are apparent in various beta subunits of glycoprotein hormones and the regions around residues 51 through 57 and 75 through 80 in the beta structures are suggested to be involved in interaction with the common alpha subunit (Szkudlinski et al., 2002).
Effects of Drugs on TSH Concentrations
This subject has been reviewed (Daminet and Ferguson, 2003; Ferguson, 2007). Drugs with significant effects on the thyroid axis include glucocorticoids and barbiturates. However, suppression of TSH concentrations by glucocorticoids have not been observed, probably due to the lack of sensitivity of the TSH assay.
Two studies (Daminet et al., 1999; Gaskill et al., 1999) have examined the effects of phenobarbital on thyroid function tests. Daminet and coworkers prospectively examined the effect of a 3-week course of phenobarbital on TT4, free thyroxine by equilibriumdialysis (FT4D), and TSH, and observed no significant alteration of these values over this time frame. Gaskill and coworkers, in an effort to dissect out the effects of phenobarbital, documented TT4 and TSH in 78 dogs receiving phenobarbital and compared them with 48 untreated epileptic dogs. Of the dogs on phenobarbital, 40% had low TT4 and 7% had elevated TSH, while only 8% of untreated dogs had low TT4 and none had elevated TSH. Of the latter group, only dogs with recent seizure activity had low TT4 values. The investigators found no effect of phenobarbital on serum binding of T4. The mean serum TT4 was significantly lower, and mean serum TSH significantly higher in the phenobarbital-treated group. As with the subacute study (Daminet et al., 1999), there did not appear to be a correlation between phenobarbital dosage or duration of treatment and the serum TT4 and TSH concentrations.
Dopamine and dopamine agonist bromocryptine were shown to suppress serum TSH in patients with selective pituitary resistance to thyroid hormone (Ohzeki et al., 1993). Other drugs that suppress TSH either at the level of hypothalamus or pituitary include retinoids (vitamin A derivatives) and somatostatin analogues (reviewed by Haugen, 2009).