Thyrotropin-releasing hormone: Clinical Studies and Safety
General Description
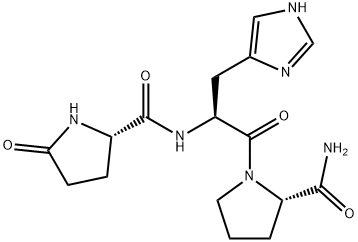
Thyrotropin-releasing hormone (TRH) (l-pyroglutamyl-l-histidyl-l-prolinamide; TRH; protirelin), the smallest known peptide hormone, has central effects unrelated to endocrine action via the pituitary-thyroid axis. Recently, TRH was successfully used for treating children with such neurologic disorders as intractable epilepsy, spinal muscular atrophy, and cerebellar ataxia. The results of animal experiments suggested that TRH modulates the release and turnover of monoamines and has an antiepileptic action.

Clinical Studies
Thyrotropin-releasing hormone and its receptors (TRH-R1, TRH-R2) are distributed in neurons throughout the CNS, especially in limbic and sensory loci. TRH and its analogues are known to modulate the central actions of classical neurotransmitters such as dopamine, norepinephrine, serotonin, acetylcholine, GABA, and glutamate by poorly defined mechanisms. Potentially, one or several of these neurotransmitter interactions, as well as others, could be involved in the pharmacological effects of TRH. Clinically, TRH has shown efficacy in affective and cognitive disorders, alcohol withdrawal, and selected neurodegenerative disorders, such as intractable epilepsies, progressive cerebellar ataxia, and acute neurotrauma.
Little is known about the mechanism of action of centrally acting TRH. The apparent prolonged duration of the effect of TRH reported clinically suggests a novel mechanism of action. Presently, two high-affinity TRH receptors have been cloned. TRH-R1 is highly expressed in limbic structures, whereas TRH-R2 is expressed primarily in sensory processing regions. Both are members of the G-protein coupled receptor (GPCR) superfamily that uses the Gaq/11 subunit for effector coupling to phospholipase C, resulting in diacylglycerol (DAG) and inositol tris-phosphate (IP3) as second messengers mediating cellular events through protein kinase C phosphorylation and Ca++ respectively. Glutamate acts through ionotropic and metabotropic (GPCR) glutamate receptors (mGluRs). Whereas ionotropic receptors (NMDA, AMPA, kainate) mediate fast neurotransmission, mGluRs (mGluR1–8) generate somewhat slower and longer-lasting changes in the heterotrimeric signaling cascades in neurons. These glutamate receptors are distributed throughout the subfields of the hippocampal formation. Early studies by Renaud and coworkers reported a selective inhibition of glutamate-stimulated neuronal activity by TRH. At the same time, Koenig and colleagues showed that TRH could inhibit glutamate-stimulated Ca++ uptake in cultured cortical cells.
Biological functions
The tripeptide, thyrotropin-releasing hormone, the first hypothalamic-releasing hormone to be isolated, subserves a key role in feedback regulation of the hypothalamic-pituitary-thyroid axis in maintaining thyroid homeostasis. TRH stimulates the release of prolactin in addition to thyroid-stimulating hormone, and these effects are used diagnostically to test the integrity of the hypothalamus and pituitary. The discovery of thyrotropin-releasing hormone, TRH mRNA, and associated receptor(s) and metabolic enzymes outside the hypothalamus quickly established the significance of TRH in several nonendocrine functions. TRH and its analogs are known to modulate the actions of such classical neurotransmitters as acetylcholine, noradrenaline, dopamine, serotonin (5-hydroxytryptamine), and glutamate, indicating a broad range of neurophysiologic functions for TRH. A role for TRH has been explored in affective and cognitive disorders and some neurodegenerative disorders, such as amyotrophic lateral sclerosis, progressive cerebellar ataxia, acute neurotrauma, and alcohol withdrawal.
Side effects
TRH and its analogs have been associated with few side effects. Clinical studies using various intrathecal, IV, intramuscular, subcutaneous, or parenteral doses in amyotrophic lateral sclerosis, senile dementia, women at risk for delivering premature infants, and intractable epilepsy have reported infrequent and transient side effects. These have included urinary retention, irritability, sleepiness, mild hypertension, tachycardia, appetite loss, nausea and vomiting, sweating, shivering, and cold and warm sensations. Zegher et al. performed follow-up thyroid function studies in 26 offspring 6 years after their mothers had received prenatal TRH (400 μg every 6 hours for 4 days) to improve lung maturation. Thyroid function seemed normal in all 26 children, indicating no adverse affect on the hypothalamo-pituitarythyroid axis. A recent long-term TRH trial for dementia (10 mg daily intramuscularly for 3 months) reported only three TRH-treated patients of 107 (2.8%) dropped out because of adverse side effects; this was not significantly different than the placebo group. No morbidity or mortality has been reported with TRH use, and only one report has appeared in which a patient with preexisting epilepsy had a seizure after diagnostic TRH injection. Thus, TRH and its analogs seem safe when used in a wide age group ranging from prenatal exposure to elderly patients.
Reference
1. Kubek MJ, Garg BP. Thyrotropin-releasing hormone in the treatment of intractable epilepsy. Pediatr Neurol. 2002;26(1):9-17.
2. Takeuchi Y. TRH (Protirelin): Role in the treatment of epilepsy. CNS Drugs. 1996; 6: 341-350.
You may like
Related articles And Qustion
See also
Lastest Price from Thyrotropin-releasing hormone manufacturers

US $0.00-0.00/KG2025-10-08
- CAS:
- 24305-27-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T
US $2.00-5.00/kg2025-06-25
- CAS:
- 24305-27-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg