The Versatile World of Triphenylmethanol: Applications and Innovations
Introduction
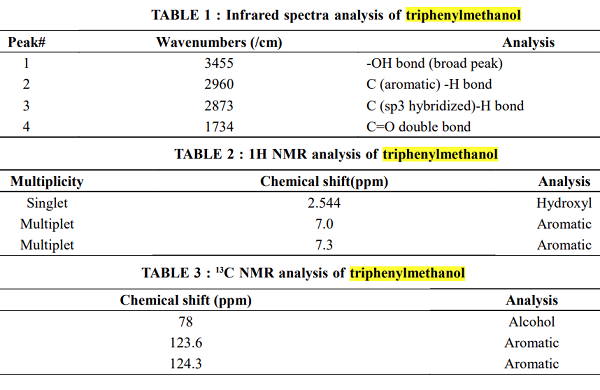
Triphenylmethanol, with the molecular formula (C₆H₅)₃COH, is an aromatic alcohol featuring a central carbon atom bonded to three phenyl rings. This compound has gained prominence in organic chemistry due to its intriguing structure and its significant utility in various applications. The combination of the phenyl groups with the hydroxyl group imparts unique chemical properties that make it a valuable substance in both industrial and research settings.1
Synthesis
The synthesis of triphenylmethanol has been achieved through various methods, each tailored to maximize yield and purity while minimizing environmental impact:
Traditional Methods
The most common synthesis involves the Friedel-Crafts alkylation of benzene using triphenylmethane and a Lewis acid catalyst like aluminum chloride (AlCl₃). This reaction typically produces high yields of triphenylmethanol. The mechanism involves the formation of a carbocation intermediate that reacts with benzene to form triphenylmethane, which is then oxidized to it.2
Modern Advances
Recent advancements focus on improving the efficiency and environmental footprint of this synthesis. For instance, researchers are exploring the use of greener catalysts, such as recyclable metal-organic frameworks (MOFs), and employing solvent-free or aqueous conditions to reduce the use of hazardous solvents. These approaches align with the principles of green chemistry and aim to make the synthesis of triphenylmethanol more sustainable.3
Applications
Organic Synthesis
Triphenylmethanol is a versatile intermediate in organic synthesis with several notable applications:
Triphenylmethane Dyes: One of the primary applications of triphenylmethanol derivatives is in the synthesis of triphenylmethane dyes. These dyes, which include Crystal Violet and Malachite Green, are used extensively in textiles, biological staining, and pH indicators due to their vivid colors and high stability.
Pharmaceutical Synthesis: In pharmaceutical chemistry, triphenylmethanol serves as a precursor for various drugs. Its derivatives are used in the development of compounds that target specific biological pathways, including enzyme inhibition and receptor modulation.
Polymer Chemistry: Triphenylmethanol is employed in the synthesis of specialty polymers. Its structure allows for the creation of high-performance materials with desirable properties, such as enhanced thermal stability and mechanical strength.
Pharmaceutical Industry
Triphenylmethanol and its derivatives hold considerable promise in pharmaceutical applications:
Drug Development: The triphenylmethanol scaffold is used to design and synthesize molecules with potential therapeutic effects. Researchers have explored its derivatives for their ability to modulate biological targets, including proteins and enzymes involved in disease processes.
Anti-Cancer Agents: Some triphenylmethanol derivatives exhibit anti-cancer properties by interfering with cellular pathways critical to cancer progression. Their ability to bind selectively to cancer-related proteins makes them candidates for further development as anticancer agents.
Neurological Disorders: Other derivatives are being investigated for their potential in treating neurological disorders. Their ability to cross the blood-brain barrier and interact with central nervous system targets makes them relevant in this context.
Material Science
In material science, triphenylmethanol’s derivatives contribute to the advancement of various materials and technologies:
Polymers and Plastics: Triphenylmethanol-based polymers exhibit excellent mechanical and thermal properties. They are used in the manufacture of high-performance plastics, resins, and coatings that require durability and stability.
Coatings and Composites: Triphenylmethanol derivatives are also utilized in the formulation of advanced coatings and composites. These materials find applications in industries such as aerospace, automotive, and electronics, where superior material properties are essential.
Nanotechnology: Researchers are exploring the role of triphenylmethanol in nanotechnology. Its derivatives are being studied for their potential use in creating nanoscale materials and devices, leveraging their unique chemical properties for innovative applications.4
Innovations and Future Directions
The field of triphenylmethanol chemistry is evolving, with ongoing research focusing on:
Green Chemistry: Efforts are underway to develop more sustainable synthesis methods that minimize waste and use environmentally friendly reagents. The goal is to create processes that align with the principles of green chemistry while maintaining high efficiency.
Nanotechnology and Advanced Materials: Future research is likely to explore new applications in nanotechnology, including the creation of nanostructured materials with novel properties. Additionally, triphenylmethanol's role in developing smart materials and devices will be a key area of investigation.
Pharmaceutical Innovations: Continued research into triphenylmethanol derivatives could lead to the discovery of new drugs and therapeutic agents. Advances in medicinal chemistry may uncover additional applications in treating various diseases and conditions.5
Conclusion
Triphenylmethanol's unique chemical structure and properties make it a valuable compound with diverse applications across multiple fields. From its role in organic synthesis and material science to its potential in pharmaceuticals, triphenylmethanol continues to be a subject of significant interest and research. As innovations and new technologies emerge, its applications are likely to expand, highlighting its importance in both traditional and cutting-edge chemical processes.
References:
[1] G. FERGUSON. The Structure of Triphenylmethanol, Ph3COH[J]. Acta crystallographica. Section C, Crystal structure communications, 1992, 6 1: 299-316. DOI:10.1107/S0108270191015044.[2] SAMIYAH ALHAMED. Triphenylmethanol Conjugates of Triptorelin as Anti-Lipid Peroxidation Prodrugs[C]//43 1. 2019: 1458-1465. DOI:10.4236/OJMC.2019.93003.
Related articles And Qustion
Lastest Price from Triphenylmethanol manufacturers

US $50.00-10.00/kg2025-09-02
- CAS:
- 76-84-6
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg

US $10.00/KG2025-04-21
- CAS:
- 76-84-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt