The Versatile Role of L-Alaninol in Chemical Synthesis and Pharmaceutical Applications
Introduction
L-Alaninol is a pharmaceutical intermediate compound used in the preparation of Cabotegravir and Encorafenib, an HIV-1 integrase inhibitor used for the treatment and pre-exposure prophylaxis of HIV-1 infections, and Encorafenib, a kinase inhibitor used for the treatment of specific mutations in unresectable or metastatic melanoma. L-Alaninol can be obtained by chemical synthesis or microbial preparation.

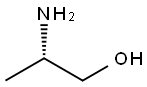
Figure 1 Characteristics of L-Alaninol
(1) A cell-free preparation was obtained from a culture of Mycobacterium avium. This preparation contained an L-alanine reductase which reduces L-alanine to L-alaninol, a constituant of the mycoside C2. This reduction is better when ATP and NADH are present in the incubation medium.
(2) The inorganic acid salt of L-alaninol ester, preferably sulphate, hydrochloride or p-toluenesulphonate, is reduced to L-alaninol by dropwise addition of a large amount, typically 3.0-5.0 times equivalent, of an aqueous NaBH4 solution at 10-40 °C, preferably at 15-25 °C.
Properties
L-Alaninol is a colourless to light yellow liquid with a faint special odour. It has a melting point of -1°C at atmospheric pressure and a boiling point of 183-185°C. It is soluble in water, ethanol and other polar solvents. L-Alaninol is the enantiomer of D-Alaninol or (R)-2-aminopropan-1-ol.
Applications
L-Alaninol is an amino acid alcohol compound widely used in the preparation of pharmaceutical and chemical products. In the field of organic synthesis, it can be used to prepare organic amine compounds. In pharmaceutical manufacturing, it is often used as a drug intermediate component, which can be used to prepare drugs such as Cabotegravir, Encorafenib and Zoliflodacin. In addition, L-Alaninol can also be used in biological activity research, the results show that L-Alaninol has anti-cancer activity, can inhibit the proliferation of melanoma cells, increase the level of cytochrome c reductase and tau-glutamyl transpeptidase.
Storage Methods
L-Alaninol should be stored in a cool, dry place away from direct sunlight and heat sources. Typically, L-Alaninol should be stored in sealed containers made of materials that will not react with alcohol, such as glass or certain plastics. In addition, whenever possible, avoid contact with strong oxidising agents and acids, which may react with L-Alaninol to produce unwanted by-products.
References:
[1] H.S. LALITHAMBA . Synthesis, solvatochromic properties, and dipole moments of Fmoc-l-alaninol[J]. Journal of Molecular Liquids, 2014, 198: 1-430. DOI:10.1016/j.molliq.2014.07.008.[2] D. SIVARAMAKRISHNA M J S. Synthesis, characterization and thermotropic phase behavior of a homologous series of N-acyl-l-alaninols and interaction of N-myristoyl l-alaninol with dimyristoylphosphatidylcholine[J]. Chemistry and Physics of Lipids, 2016, 196: 1-88. DOI:10.1016/j.chemphyslip.2016.01.001.
See also
Lastest Price from L-Alaninol manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 2749-11-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1T+
US $0.00-0.00/kg2025-04-18
- CAS:
- 2749-11-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 10mts