The toxicity of Glycidyl methacrylate
Introduction
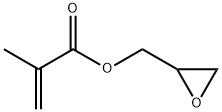
Glycidyl Methacrylate (GMA) is a clear, colourless liquid with a strong ester and fruity odour. It'sIt comprises a polymerizable methacrylate functional group on one end and a reactive epoxy group on the other. It is slightly miscible with water, soluble in most organic solvents and has relatively low volatility. Its vapour is heavier than air. It copolymerizes readily with various functional groups, such as phenols, ketones, acids and amines. The added epoxy group imparts durability, mechanical strength, optical transparency, and adhesiveness.
Uses
Glycidyl methacrylate, which has both vinyl and epoxy functions, is a reactive monomer that meets the requirements for post-polymerization modification. It is also a low-cost reagent because it is widely used for the industrial production of epoxy functional methacrylic resins to be employed as coatings and adhesives. GMA is a chemical monomer for the manufacture of epoxy polymers, acrylic and vinyl resins, dental resins and sealants, polymer coatings, adhesives, dyes and paper and beverages and wastewater treatment. In addition, the FDA classified GMA as a food contact substance A – as an internal surface thin coatg – for food storage/ packaging, bone composite materials, and hydrogel lenses.
In the pharmaceutical industry, GMA can manufacture suspensions, creams and ointments. Also, it can be used in local and systemic controlled drug delivery systems, including enterosoluble gastro-resistant coatings, transdermal systems, microencapsulation, target colon oral drug delivery, and as a system of drug administration based on a pH and ionic strength polymer responder.
Toxicity
Glycidyl methacrylate has a vinyl or highly reactive (epoxy) group, leading to genotoxicity alerts.
Studies show that dental resins made of polymers (monomers and (co)monomers) were associated with allergic reactions and adverse effects on gum and pulp chamber health. Since GMA polymerization is generally incomplete and not all methacrylate (co) monomer groups react, the residual monomers undergo a process of leaching the teeth, which can be enhanced by saliva, food or drink.
GMA is an ester of methacrylic acid. Methacrylates are used in the polymer form; however, polymers can release monomers and co-monomers into the bloodstream, reaching all organs. Genotoxicity of methacrylate monomers has special significance owing to potential serious phenotypic consequences, including cell death, mutations, cancer and a long latency period.
The general population is not directly exposed to GMA. The production of GMA takes place in closed systems and is used for resin synthesis, where occupational exposure is expected through inhalation and dermal routes. Human exposure to GMA occurs mainly among workers whose occupations are related to producing dental resins and bone compound materials and consuming finished products. Although recognized as hazardous, chemical manufacturing is controlled to avoid accidental exposure, for example, by employees in the workplace.
The concern with GMA is that it may be present in food and, consequently, affect a large number of people through drinking water processed from surface water and through fish that may accumulate this chemical. GMA can be absorbed via inhalation and dermal and gastrointestinal (GI) routes. Cough, sore throat and difficulty breathing characterize the observed symptoms, followed by exposure from the inhalation route. Dermatological conditions can be characterized by redness, pain and a burning sensation. GMA is also irritating to the eyes, causing signs similar to redness and symptoms like pain and burning sensations. GI symptoms of GMA exposure include sore throat/ throat, chest burning sensation, and abdominal pain. Chronic exposure can cause skin sensitization. In addition to GI absorption, unreacted monomers in dental resins can diffuse through the pulp chamber from dental resins or be absorbed by the lungs by inhalation and reach the systemic circulation. Through experimental studies, two species of animals of both sexes, exposed by inhalation, obtained evidence for GMA carcinogenicity. In mice from both sexes, GMA also induced nasal cavity hemangiosarcomas, bronchioloalveolar squamous cell carcinoma, peritoneal mesotheliomas, and in females, a uterine histiocytic and endometrial sarcoma.
References
[1] Bruno Jose Dumêt Fernandes, Ricardo David Couto. “Toxicological alert: Exposure to glycidyl methacrylate and cancer risk.” Toxicology and Industrial Health 36 12 (2020): 937–939.
Related articles And Qustion
See also
Lastest Price from Glycidyl methacrylate manufacturers

US $50.00-10.00/kg2025-09-02
- CAS:
- 106-91-2
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg

US $0.00/kg2025-06-05
- CAS:
- 106-91-2
- Min. Order:
- 230kg
- Purity:
- 99%
- Supply Ability:
- 20 ton