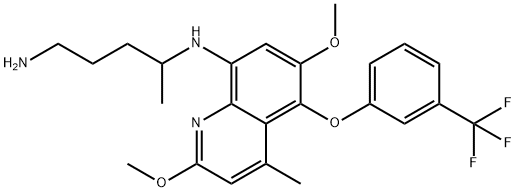
The synthesize method of tafenoquine
Overview
A century-long history in 8-aminoquinolines, the only anti-malaria drug class preventing malaria relapse, has resulted in the approval of tafenoquine by the U.S. Food and Drug Administration (FDA) and the Australian Therapeutic Goods Administration (TGA) and to date registration in Brazil and Thailand. It is an alternative anti-relapse treatment for vivax malaria and malaria prophylaxis. It is available in both preventative dose-loading regimens and radical cure dosages. Developed by the Walter Reed Army Institute of Research in the 1980s, tafenoquine (WR 238605) was described as possessing both tissue and blood schizonticidal activity. The exact mechanism of action of tafenoquine, which is a racemate, is unknown. However, evidence suggests that tafenoquine may inhibit hematin polymerization and induce apoptosis in select strains. This drug is currently being developed by GlaxoSmithKline (GSK) in collaboration with Medicines for Malaria Venture, 60° Pharmaceutical, Knight Therapeutics, and the United States Army[1].
Synthesize method
Preparation of Tafenoquine Nitroquinoline Intermediate
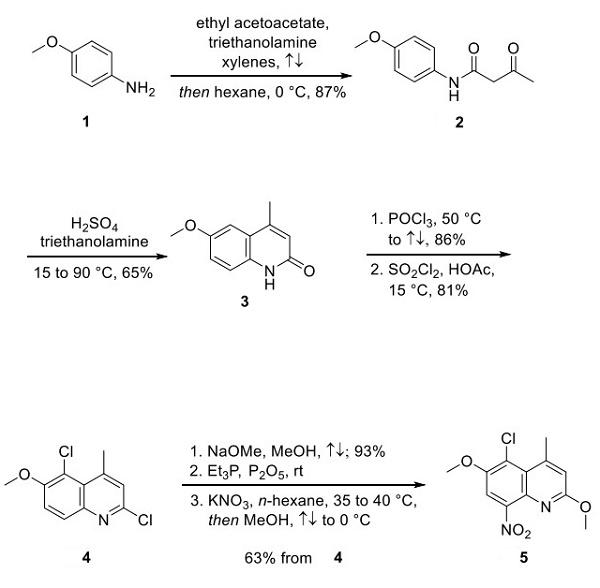
Multiple synthetic routes to tafenoquine have been reported in the patent. A 2003 patent describes the process chemistry route to access tafenoquine, and this approach is described below. P-Anisidine 1 was heated in xylenes and ethyl acetoacetate to give aniline 2 in 87% yield, which was converted to quinoline 3 via a sulfuric acid-mediated dehydrative condensation reaction. Quinoline 3 was subsequently chlorinated with phosphorus oxychloride and sulfuryl chloride to arrive at dichloroquinoline 4. Methoxide addition, treatment with triethylphosphine in base, and subsequent nitration converted compound 4 to nitroquinoline 5[2].
Synthesis of Tafenoquine
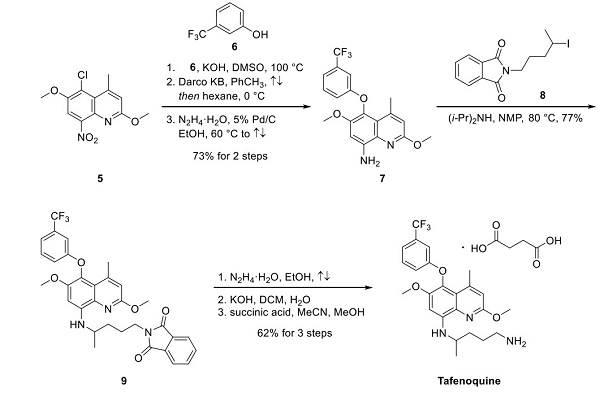
This highly activated quinoline was subjected to a substitution reaction with phenol 6 under basic conditions. Next, treatment with Darco KB and hydrazine-promoted nitroreduction gave amine 7 in a 73% yield for the three-step sequence. Alkylation of 7 with commercial iodide 8 was performed in a mixture of warm NMP and diisopropylamine, followed by hydrazine-mediated conversion of the phthalimide to the corresponding primary amine. Sequential exposure to potassium hydroxide and succinic acid completed the synthesis of tafenoquine in 63% from compound 9.
References
[1] Cindy S Chu. “Tafenoquine: a toxicity overview.”Expert Opinion on Drug Safety 20 3 (2021): 349-362.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.