The synthesis method of Apixaban
Introduction
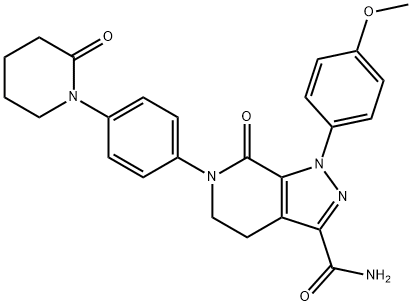
Apixaban, chemical name is 1-(4-p-methoxy-phenyl)-7-oxo-6-[4-(2-oxo-piperidine-1-yl) phenyl]-4,5,6,7-tetrahydrochysene-1H-pyrazoles [3,4-c] pyridine-3-carboxamide, is a new oral anticoagulant with a direct and specific inhibitory action on FXa and with demonstrated safety and efficacy in the prophylaxis and treatment of venous thromboembolism (VTE) in several clinical trials involving thousands of patients.
Apixaban (BMS-562247, pM-460) is a direct selective and reversible inhibitor of FXa. Apixaban binds to the active site on FXa with an elevated affinity (Ki =0.08 nM) and exerts its anticoagulant and antithrombotic mechanisms independently of antithrombin. Apixaban is a direct drug that does not require previous biotransformation to become active[1].
Indication
Apixaban is indicated for reducing the risk of stroke and systemic embolism in patients who have nonvalvular atrial fibrillation, prophylaxis of deep vein thrombosis(DVT) leading to pulmonary embolism(PE) in patients after a hip or knee replacement surgery, and treatment of DVT and PE to reduce the risk of recurrence.
Synthesis
Based on its usefulness, it is essential to explore the synthesis method of Apixaban.
An invention discloses a synthetic method of novel Apixaban. The method comprises the following steps: (i) hydrolyzing an Apixaban precursor compound (II) to obtain a carboxylic acid product; and (ii) mixing the carboxylic acid product obtained in step (i) with ethyl chloroformate, reacting in the presence of diisopropylethylamine at the temperature of 0-5 DEG C for 3-5 hours; then introducing ammonia gas and reacting to obtain an ammonolysis product, namely, Apixaban. By adopting the method for preparing Apixaban by using the Apixaban precursor compound (II), the yield of Apixaban can be up to 93 per cent. In the entire synthesis route, the minimum yield in each step is over 76 per cent at least, and the total yield is about 33 per cent. In the entire process, the use of precious catalysts or auxiliary reagents is avoided.
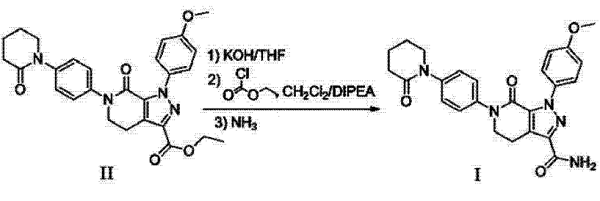
Add 4.88g (10mmol) of ethyl 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylate (compound II), 50ml of THF, and 0.56g (10mmol) of KOH to the reaction flask. After addition, heat to 50℃ and react for 3-5 hours. Follow the reaction by LCMS until the reaction of compound II is complete. Cool down to 0-5℃ in an ice-water bath, slowly add 1mol/L HCl to the reaction solution to adjust the pH to about 6, stir thoroughly until the precipitate (i.e., the corresponding carboxylic acid product) precipitates, filter, and vacuum dry at 50℃[2].
Add the above vacuum-dried compound to the reaction flask, then add 50ml of CH2Cl2 and 1.29g (10mmol) of diisopropylethylamine. After addition, adjust the temperature to 0-5℃ and add 1.08g (10mmol) of ethyl chloroformate dropwise. Keep 0-5℃ to react for 3-5 hours to generate mixed anhydride. Follow the reaction by TLC until the reaction is complete. Keep 0-5℃ and pass ammonia gas into the reaction flask. Follow the reaction by TLC until the reaction is complete. The organic layer was washed with H2O and saturated sodium chloride solution, dried over anhydrous sodium sulfate for 4 h, filtered, and the filtrate was concentrated in vacuo to obtain 4.27 g of a solid compound; molar yield: 93%, purity 99.3%, i.e., Apixaban.
References:
[1] L PUJADAS-MESTRES. Apixaban in the prevention of stroke and systemic embolism in nonvalvular atrial fibrillation.[C]//49 7. 2013. DOI:10.1358/dot.2013.49.7.1980498.Related articles And Qustion
See also
Lastest Price from Apixaban manufacturers

US $5.00-0.50/KG2025-05-30
- CAS:
- 503612-47-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/KG2025-04-21
- CAS:
- 503612-47-3
- Min. Order:
- 10g
- Purity:
- 98%-102% HPLC
- Supply Ability:
- 10kg