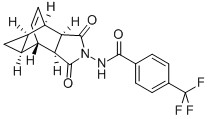
The synthesis method and effect study of Tecovirimat
Overview
Tecovirimat, developed by SIGA Technologies and the United States Department of Health and Human Services Biomedical Advanced Research and Development Authority, is the first oral treatment for smallpox. Although smallpox was eradicated due to effective vaccination practices, many people worldwide are now unvaccinated. Because health authorities believe that a single case of the disease could trigger a global health emergency, identifying this small molecule therapy was intended to serve as a countermeasure.
Synthesize method
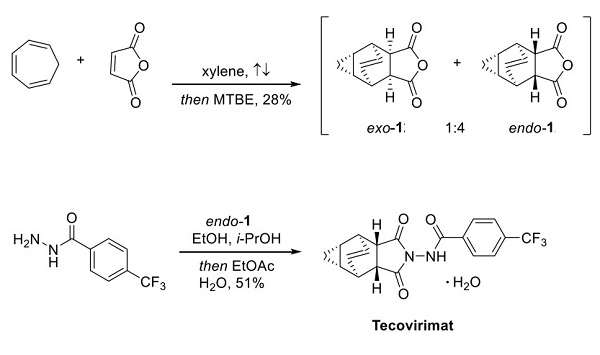
An efficient, three-step synthetic route to tecovirimat, shown above, was demonstrated[1]. DielsAlder cycloaddition of cycloheptatriene and maleic anhydride in refluxing xylenes formed the fused tricyclic core in a single step and a 4:1 product ratio, favoring the expected 1-endo isomer, which could be isolated by recrystallization from MTBE. The reaction of this fused anhydride with 4-(trifluoromethyl)benzohydrazide in a mixture of alcohols proceeded without inversion or epimerization of the stereocenters. Recrystallization from EtOAc/water facilitated the isolation of tecovirimat as the hydrate[1].
Effect study
Initiated by a biodefense effort from the National Institute of Allergy and Infectious Disease, tecovirimat was identified by screening libraries of over 300,000 known compounds for their ability to interfere with the replication of vaccinia or cowpox. The mechanism of action of tecovirimat likely involves the F13L gene of the vaccinia virus, which encodes a membrane protein required for extracellular virus production. Tecovirimat was approved under the USFDA’s Animal Rule, as clinical studies in humans were not ethical or feasible. This led to several challenges, as the variola virus that causes smallpox has only been observed in humans. As a result, it was necessary to use three animal models: rabbitpox virus in rabbits, ectromelia virus (mousepox), and monkeypox in non-human primates. Tecovirimat became part of the U.S. Government’s Strategic National Stockpile upon approval.
References
[1] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
See also
US $0.00-0.00/KG2025-11-28
- CAS:
- 816458-31-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $1.00/g2025-03-28
- CAS:
- 816458-31-8
- Min. Order:
- 1g
- Purity:
- 99
- Supply Ability:
- 1000