The synthesis and history of plazomicin
Description
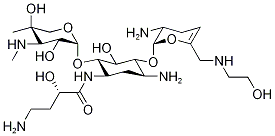
Plazomicin (formerly ACHN 490) is a novel aminoglycoside that has been developed to target multidrug-resistant (MDR) Enterobacteriaceae, including organisms capable of producing aminoglycoside-modifying enzymes (AMEs), extended-spectrum β-lactamases (ESBLs), and carbapenemases[1]. These resistant pathogens may lead to severe bacterial infections, including but not limited to nosocomial pneumonia or bacteremia, which have become problematic worldwide.
As a structural derivative of the anti-infective aminoglycoside sisomycin, plazomicin is a neoglycoside that is highly active against various bacterial pathogens, including many of the Gram-negative rods implicated in cUTIs. Plazomicin is unaffected by most aminoglycoside-modifying enzymes and retains activity against multidrug-resistant (MDR) isolates known as carbapenem-resistant Enterobacteriaceae (CRE).
History
Initially discovered by California-based Ionis Pharmaceuticals and later developed by Achaogen, plazomicin was approved by the USFDA in 2018 for the treatment of patients 18 years of age or older with complicated urinary tract infections (cUTI), including pyelonephritis. The drug, a next-generation aminoglycoside delivered by injection, was acquired by Cipla as part of an auction of Achaogen assets after the American firm filed for Chapter 11 bankruptcy.
Synthetic method
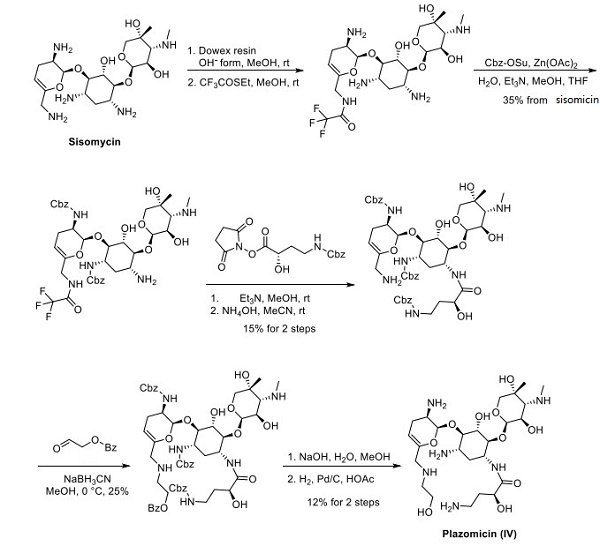
Two synthetic routes to ACHN 490 have been reported; both originate from commercial sisomicin and vary only by differential protection of the sisomicin amines. Sisomicin was treated with an ionexchange resin, which furnished trifluoroacetamide after reaction with ethyl trifluorothioacetate. Zinc acetate and benzyloxycarbonyl succinimide provided the corresponding Cbz-protected intermediate in a 35% yield from sisomicin. Amide coupling and removal of the trifluoroacetate group yielded glycoside, which then underwent reductive amination, benzoyl ester cleavage, and global Cbz removal under hydrogenative conditions to give rise to plazomicin[2].
Pharmacokinetics
ACHN 490, as with other aminoglycoside antimicrobials, is poorly absorbed and must be administered parenterally. The mean Vd of plazomicin in healthy adults and those with complicated urinary tract infections (cUTIs) was 18 and 31 L, respectively. A mean maximum serum concentration (Cmax) of 51 mg/L and an area under the concentration-time curve (area under the curve [AUC]) of 226 mg ∙ h/L was observed following a single intravenous dose (15 mg/kg) of plazomicin in patients with cUTI[3]. The binding of plazomicin to plasma proteins is approximately 20%. In addition, Plazomicin is primarily excreted by the kidneys. This drug is not metabolized appreciably and does not induce or inhibit cytochrome P450 drug-metabolizing enzymes. The mean serum elimination half-life of plazomicin is approximately 3.5 h in patients with normal renal function.
References
[1] Kristy M Shaeer. “Plazomicin: A Next-Generation Aminoglycoside.” Pharmacotherapy 39 1 (2019): 77–93.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
[3] L. Saravolatz, G. Stein. “Plazomicin: A New Aminoglycoside.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 20 1 (2019).
See also
US $0.00/kg2025-04-15
- CAS:
- 1154757-24-0
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons