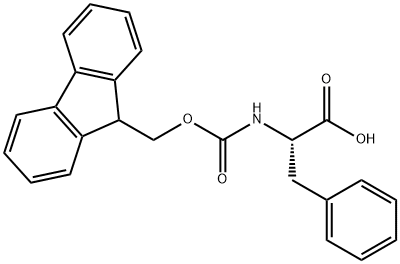
The synthesis and applications of Fmoc-Phe-OH
General description
Fmoc-Phe-OH, formally referred to as 9-Fluorenylmethoxycarbonyl-L-phenylalanine, is a pivotal compound in the realm of peptide synthesis. The Fmoc group, serving as a protective moiety for amino acids, plays an indispensable role in peptide synthesis by shielding the amino group through a stable amide bond, thereby preventing undesired side reactions during peptide bond formation. The Fmoc group is particularly favored in solid-phase peptide synthesis (SPPS) due to its robustness under basic conditions and its capability to be selectively removed under mild alkaline conditions without affecting the integrity of the peptide backbone. Consequently, Fmoc-Phe-OH serves as an essential building block in the process of peptide synthesis.
Synthesis

Fig. 1 The synthesis route of Fmoc-Phe-OH
Scheme 1:Add N, N-Diisopropylcarbodiimide ( 31.2 μL, 0.2 mmol, 3 equiv ) and DMAP ( 2.4 mg, 0.02 mmol, 0.3 equiv ) to a solution of support ( 50 mg, 0.067 mmol, 1 equiv ) and Fmoc-AAOH ( 1.2 equiv ) in DMF ( 0.5 mL ) .Allow the reaction to stir at room temperature for 6 h. Precipitate with diethyl ether, followed by centrifugation. The synthesis route is shown in Fig. 1[1].

Fig. 2 The synthesis route of Fmoc-Phe-OH
Scheme 2:Add thiophenol (61 ml, 0.595 mmol, 2.0 equivalents) and cesium carbonate (290 mg, 0.890 mmol, 3.0 equivalents) to a stirred solution of N-Fmoc phenylalanine (1-nosyl-5-nitroindol-3-yl)methyl ester (0.296 mmol) in acetonitrile (1.5 ml) at room temperature. After stirring for 1.5 hours, partition the reaction mixture between ether and a 3 N aqueous hydrochloric acid solution. Wash the organic layer with brine. Dry the organic layer over sodium sulfate. Filter the organic layer. Remove the solvent in the vacuum. Purify the residue by flush silica gel column chromatography (toluene to 1% methanol in dichloromethane). The synthesis route is shown in Fig. 2.

Fig. 3 The synthesis route of Fmoc-Phe-OH
Scheme 3: Stir a solution of the prenyl ester (1 mmol) and TMSOTf (0.02-0.03 mmol) in dichloromethane (3 mL) at room temperature for 1 to 5 hours. Evaporate the volatile materials under vacuum. Purify the residue directly by crystallization or column chromatography on silica gel. The synthesis route is shown in Fig. 3[1].

Fig. 4 The synthesis route of Fmoc-Phe-OH
Scheme 4: Add an aqueous 15% solution of NaHCO3 (15 mL) to a solution of alcohol (1.11 g) in acetone (50 mL) with stirring at 0°C.Add a solid NaBr (0.1 g, 1 mmol) and TEMPO (0.015 g, 0.1 mmol) to the reaction mixture. Add trichloroisocyanuric acid (2.32 g, 10.0 mmol) slowly to the reaction mixture within 20 minutes at 0°C. After addition, warm the reaction mixture to room temperature. Stir the mixture until the reaction completes. Add 2-propanol (3 mL) to the mixture. Filter the mixture on Celite. Concentrate the mixture under a vacuum. Treat the mixture with 15 mL of a saturated solution of Na2CO3. Wash the aqueous phase with portions of AcOEt.Treat the aqueous phase with 1 N HCl.Extract the aqueous phase twice with AcOEt.Dry the organic layers over Na2SO4. Evaporate the solvent. Isolate the product. The synthesis route is shown in Fig. 4[2].
Applications
The utility of Fmoc-Phe-OH extends beyond its role in solid-phase peptide synthesis (SPPS). It is also employed in the synthesis of peptide libraries, which play a crucial role in drug discovery and development. Peptide libraries facilitate the identification of peptides with potential therapeutic applications, enabling rapid screening against various biological targets. Additionally, Fmoc-Phe-OH is utilized for peptide modification, including labeling or conjugation to other molecules, essential for studying peptide function and structure. Moreover, Fmoc-Phe-OH finds application in the synthesis of cyclic peptides that have gained attention due to their stability and efficacy as therapeutics. The Fmoc strategy enables the formation of cyclic peptides through orthogonal protection strategies, providing a versatile tool for creating complex peptide structures. In conclusion, Fmoc-Phe-OH serves as a cornerstone in the field of peptide synthesis by offering a versatile and efficient approach to assembling peptides. Its applications range from producing simple linear peptides to complex cyclic and modified ones, highlighting its significance in both research and therapeutic development.
References
[1] Perchyonok, V. T., et al. (2008). Facile and selective deallylations of esters under "aqueous" free-radical conditions. Synlett, (8), 1233-1235.
[2] De Luca, L., et al. (2003). Trichloroisocyanuric/TEMPO oxidation of alcohols under mild conditions: A close investigation. Journal of Organic Chemistry, 68(12), 4999-5001.
Related articles And Qustion
See also
Lastest Price from Fmoc-Phe-OH manufacturers

US $0.00-0.00/g2025-04-21
- CAS:
- 35661-40-6
- Min. Order:
- 10g
- Purity:
- 98%min
- Supply Ability:
- 10KG
US $0.00-0.00/kg2025-04-04
- CAS:
- 35661-40-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton