The side chain synthesis of boceprevir
Boceprevir is a peptidomimetic protease inhibitor with four moieties, P1–P3 and a Cap, where P1 is a racemic β-aminoamide, P2 is a chiral dimethylcyclopropylproline analog, P3 is (S)-tert-leucine, and Cap is a tert-butylcarbamoyl group. The 3-azabicyclo[3.1.0]hexane structure of P2 adopts a constrained conformation, so that the gem-dimethyl group has a fixed angle with respect to the bicyclic ring structure. The incorporation of the 3-azabicyclo[3.1.0]hexane moiety results in a 1000-fold increase in NS3 protease binding over proline in a pentapeptide scaffold. When boceprevir binds to the NS3 protease, the P2 moiety interacts with four amino acid residues at the active site.
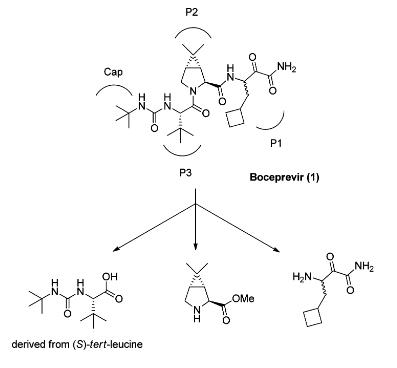
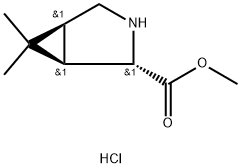
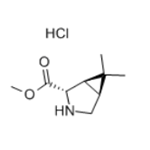
(1R,2S,5S)-methyl 6,6-dimethyl-3-aza-bicyclo[3.1.0]hexane-2-carboxylate hydrochloride is the most expensive key intermediate in the side chain synthesis of boceprevir. The synthesis of boceprevir from (1R, 2S, 5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate methyl ester hydrochloride is simple and beneficial to industrialization production characteristics.
Synthesis Method
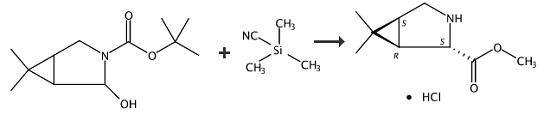
Step 1
Synthesis Example of 3-tert-Butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carbonitrile A solution prepared by mixing 0.20 g (0.88 mmol) of 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-ol obtained in the same manner as in Example 3, and 0.19 g of trimethylsilyl cyanide and 14 ml of methyl tert-butyl ether was cooled down to -78°C., and 0.27 g of a boron trifluoride-diethyl ether complex was added dropwise at -78 to -68°C. The mixture was thermally insulated at the same temperature for 3 hours, then, heated up to -40°C. and thermally insulated at the same temperature for 4 hours. To this, 5.3 g of a 7% sodium hydrogen carbonate aqueous solution was added, and the mixture was heated up to 25°C. before liquid-partitioning. The aqueous layer was further subjected to an extraction and liquid-partitioning operation twice using 10 ml of methyl tert-butyl ether. The resultant organic layers were all mixed, and to this was added sodium sulfate and mixed, then, solid was removed by filtration. The resultant solution was partially concentrated under reduced pressure to distill off the solvent. The yield with respect to 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-ol was 83.2%. The results of 1H-NMR (CDCl3) of 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carbonitrile are shown below. 0.20 g of a solution containing 3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carbonitrile 0.17 g (0.73 mmol) was obtained.
Step 2
Synthesis Example of methyl (1R,2S,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carboxylate hydrochloride 1.00 g (4.23 mmol) of (1R,2S,5S)-3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carbonitrile, and 7.00 g of a methanol solution containing 20 wt % of hydrogen chloride were mixed at 20°C. to 30°C., then, thermally insulated at the same temperature. Disappearance of the raw material compounds was confirmed, then, the reaction liquid was concentrated under reduced pressure to distill off the solvent. The yield of methyl (1R,2S,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carboxylate hydrochloride with respect to (1R,2S,5S)-3-tert-butoxycarbonyl-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carbonitrile was 63.1%. 1.06 g of an oily substance containing methyl (1R,2S,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-carboxylate hydrochloride 0.55 g (2.67 mmol) was obtained.
Boceprevir Intermediates Supplier: Shanghai QiXin New Materials Technology Co.,Ltd
See also
Lastest Price from Boceprevir InterMediates manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 565456-77-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton

US $0.00/kg2025-03-07
- CAS:
- 565456-77-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons