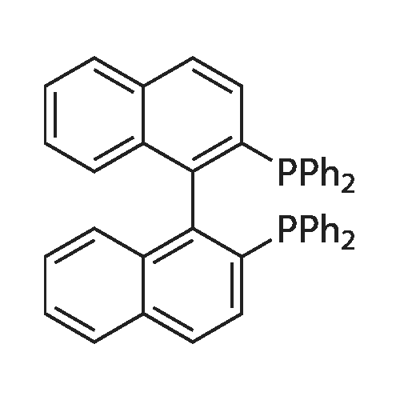
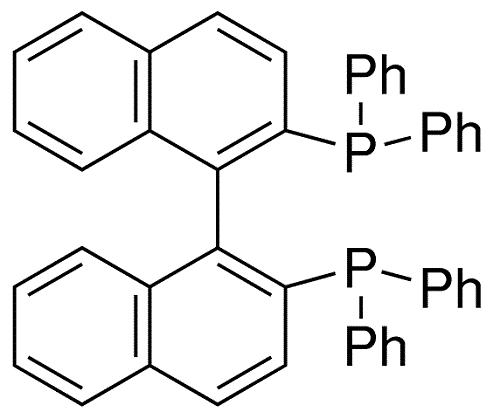
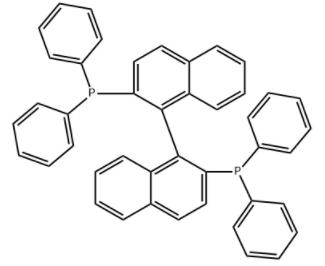
The review of 1.1'-Binaphthyl-2.2'-diphemyl phosphine
Background
BINAP is an abbreviation for the organic compound 2,2'-bisdiphenylphosphino-1,1'-binaphthyl. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphine naphthyl groups linked at the 1 and 1' positions. There are no chiral atoms in this C2-symmetric framework (see Axial Chirality). Due to steric hindrance, the racemization barrier of the bisnaphthalene rings is high and thus restricts the free rotation of the chemical bond connecting the two naphthalene rings (see atropisomers). The dihedral angle formed by the plane of its naphthalene ring is about 90˚.
BINAP complex catalysts
In 1980, the metal catalyst of BINAP ligand synthesized by Prof. Ryoji Noyori and his collaborators could precisely distinguish the enantiomeric atoms, groups or enantioplanes in latent chiral molecules, so that the synthetic purity of chiral molecules was greatly improved. Improve, especially the hydrogenation of latent chiral olefins or ketones by BINAP-Ru complex catalysts. The above-mentioned catalytic asymmetric synthesis technology only needs to use a very small amount of asymmetric catalyst to produce a large number of chiral compounds, and the catalytic efficiency is extremely high. Replacing the transition cluster metal Ru(II) with rhodium Rh(I) also proved to have the same catalytic effect. Since then, other high-efficiency catalysts have been developed one after another. These hydrogenation reactions can obtain high optical yields and chemical yields, and some reactions have been successfully used in industrial production, such as Ru(II)-BINAP complexes are also used as an important intermediate in the industrial production of antibiotic levofloxacin.
Benefiting from the research of Professor Ryoji Noyori, asymmetric hydrogenation catalytic chemistry plays a very important role in the synthesis and preparation of pharmaceuticals, agricultural products, seasonings and fragrances, as well as new and advanced materials. The efficiency of chemical synthesis using artificial molecular catalysts is comparable to that of natural enzyme-catalyzed reactions, and in some applications it even exceeds that of natural enzyme-catalyzed reactions.
In organic chemistry, enantioselective reactions can be carried out using BINAP complex catalysts with ruthenium, rhodium and palladium. Pioneers in this field, Ryoji Noyori and his colleagues discovered that the complex of rhodium and BINAP is very efficient for the synthesis of (-)-menthol.
This synthesis method was first industrialized by Takasago Electric Co., Ltd. International. Also because of this work, Ryoji Noyori won the 2001 Nobel Prize in Chemistry.
The first case of NH-vinylation of sulfoximines was described by Dehli and Bolm in 2004. In the presence of a palladium catalyst, BINAP (1.1'-binaphthyl-2.2'-diphenyl phosphine), and tBuONa, Nvinyl sulfoximines were obtained in high yields from the reaction of NH-sulfoximines with vinyl bromides(Picture 1)
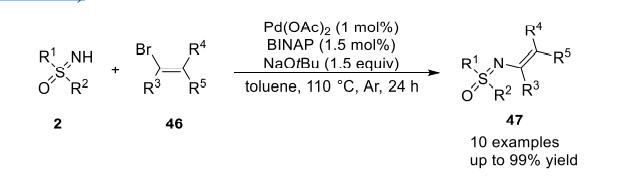
Picture 1 The reaction of NH-sulfoximines with vinyl bromides[1]
The synthesis of BINAP
BINAP can be prepared from the di-trifluoro derivative of BINOL (1,1'-bi-2-naphthol). Enantiomers of R configuration, S configuration or their racemates are all commercially available reagents. BINAP conjugated with rhodium is a widely used chemoselective hydrogenation catalyst. BINOL can be reacted with chlorodiphenylphosphine to give phosphoric acid and diphenyl-[1,1'-binaphthyl]-2-2'-diol ester (BINAPO).
BINAP ligands and their Ru complexes
Miyashita et al. developed another landmark ligand, the axial chiral biaryl bidentate phosphine ligand BINAP [1]. Initially, this ligand was used in the Rh-catalyzed asymmetric hydrogenation of α-dehydroamino acids and their derivatives, showing excellent enantioselectivity (up to >99% ee). However, the reactivity of Rh-BINAP catalysts in the asymmetric hydrogenation of such substrates is not high, and the substrates of this catalyst are not widely applicable. What really made BINAP ligands widely available was the discovery of the Ru-BINAP complex. In 1986, Noyori and Takaya et al. developed the classical Ru-BINAP catalyst , which showed excellent reactivity and enantioselectivity in the asymmetric hydrogenation of a series of functionalized olefins.
In the mid-1990s, another major breakthrough in research on BINAP ligands was the discovery of Ru-BINAP/bisamine complexes . Ohkuma et al. found that the Ru/BINAP-Dpen and Ru/BINAP/Daipen complexes catalytic systems have ultra-high reactivity and enantioselectivity in the asymmetric hydrogenation of simple ketones. When the substituent of the P atom on the ligand in the Ru/BINAP/Daipen complex is 3,5-dimethylphenyl, the complex exhibits superior performance in the asymmetric hydrogenation of a series of ketones. High reactivity and enantioselectivity. In the asymmetric hydrogenation of acetophenone, the highest turnover number (TON) of Ru/BINAP/Daipen catalyst exceeds 1 000 000, and the highest turnover frequency (TOF) exceeds 600/s. In addition, the catalyst is widely used, which is characterized by: ① high reactivity and enantioselectivity; ② wide substrate application range; ③ selective hydrogenation of carbonyl groups while retaining C=C bonds.
Reference
1 Wang C, Albrecht M, Bolm C. NH-Functionalization of Sulfoximines by Using Hypervalent Iodine Reagents[R]. Fachgruppe Chemie, 2021.
Related articles And Qustion
See also
Lastest Price from 1.1'-Binaphthyl-2.2'-diphemyl phosphine manufacturers

US $0.00/G2025-04-21
- CAS:
- 98327-87-8
- Min. Order:
- 100G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month

US $0.00-0.00/KG2025-04-21
- CAS:
- 98327-87-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt