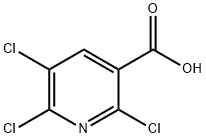
The reactions of 2,5,6-tetrachloropyridine
Background
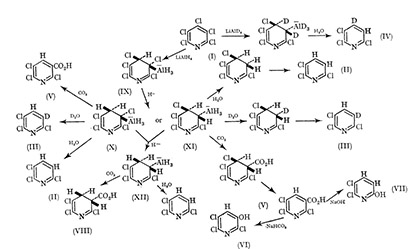
It has been reported that on treatment with lithium aluminium hydride pentafluoropyridine gives 2,5,6-tetrafluoropyridine and that further reduction occurs at the 2-position as expected from a hydride attack[1-3]. By contrast, we observed that pentachloropyridine (Ⅰ), on stirring with LiXH, at room temperature for 16 hr. in ether gives 2,3,6- trichloropyridine (Ⅱ) (go”,.), together with small amounts of the known 2,3,5,6-, 2,3,4,6-, and 2,3,4,5-tetrachloropyri- dine 2,3,6-Trichloropyridine (isolated by preparative g.1.c.) showed two doublets in the n.m.r. at T 2.77 (3-H, J 8-; i c./sec.) and at T 2.27 (4-H). 11; an effort to elucidate the nature of the reduction at the %position we treated the reaction mixture with D,O, which gave a product (Ⅲ), identical (i.r., m.p.) to (Ⅱ), but giving a much simplified n.m.r. spectrum with the high-field doublet (cf. above) absent and the low-field signals collapsed to a broad singlet (T 2.27). Compound (Ⅲ) was, therefore, 3-delxterio-2,5,6-trichloropyridine[4]. Conversely, reduction of (Ⅰ) with LiAlD,, followed by hydrolysis gave 4-deuterio2,3,6-trichloropyridine (Ⅳ), as inferred from its n.m.r. spectrum.
Reductions of 25,6-tetrachloropyridine with LiAlH
Similar reductions of 2,5,6-tetrachloropyridine or 4- deuterio-2,5,6-tetrachloropyridine, with LiAlH, led to a mixture of the 2,3,6- (7574) and the 2,3,5-trichloro-pyridine (257,)) or the respective 4-deuterio-compound[5-6]. Again, deuteriation studies confirmed that replacement of the 3- chlorine atom was not occurring via conventional hydride attark. Whereas only the trichloropyridine (Ⅱ) is obtained from pentachloropyridine (Ⅰ) with LihlH, ,the formation of both the 2,3,6- (Ⅱ) and the 2,3,5-trichloro-pyridine from 2,5,6- tetrachloropyridine under the same conditions suggests that the reduction of (Ⅰ) proceeds by a concerted attack on the 3- and the 4-positions rather than by two independent steps. Moreover, the absence of deuterium scrambling in these reactions precludes a pyridyne mechanism. Also an insertion-elimination scheme, such as that suggested by Burton and Mettille6 to rationalize the LiAlH, reduction of various fluoro-olefins is accompanied by hydrogen evolution, unlike our reactions.
Reaction with carbon dioxide
On the basis of our deuteriation experiments we suggested an organometallic intermediate which would be expected to react with carbon dioxide[7-8]. In fact, when the reaction mixture was poured on to solid CO, the crude product contained only a little trichloropyridine (Ⅱ), but showed two strong resonances in the n.m.r. (CDCl,), at r 1.00 (extinguished by D,O addition) and at T 2.09, and a strong peak at 1700 cnrl in the i.r. spectrum providing evidence for the presence of 2,3,6-trichloronicotinic acid (Ⅴ) . However, attempts to isolate the acid (V) by extraction with sodium hydrogen carbonate led to 2,3,6-trichloro-5-hydroxypyridine (VI) or to 2,3-dichloro-6-hydroxypyridine (Ⅶ) with 2~-sodiuni hydroxide. Similar results in the hydrolysis of polychloropyridine carboxylic acids have been noted previously. Another product which separated from the ether extract from the carbonation experiment on standing showed typical ABX splitting [(CD 3) $01 with no aromatic signals.
Two complex bands, a quartet of lines at T 4.75, 4.85, 4.94, 5.04, and a multiplet (T 6.73-7.68) led us to assign the structure (Ⅷ), confirmed by analysis. This compound is a rare example of a 3,4-dihydropyridine, which is presumably stabilized by the electron deficiency of the polychloro-ring. It is obviously formed by LiAlH, addition across the 3-4 bond. Only a few examples of LiAlH, addition across unconjugated olefins have been reported, but many additions of substituted aluminium hydrides across both double and triple bonds yielding organo-aluminium intermediates have been observed.9 Pyridine itself undergoes LiAlH, reduction to give 1,2-dihydro- or 1,4-dihydro-pyridine1° and various pyridines with electron-withdrawing groups are similarly reduced Ⅰ, but in all cases the products readily arornat ize . replacement of the metal group as shown. This is followed by an unusual cis-elimination which aromatises the intermediate diene to yield the observed products (Ⅱ) and (Ⅲ). In support of our thesis an analogous case of cis-elimination observed by Tatlow and his co-workers in a polyfluorohexane is particularly relevant.
Picture 1 The synthetic method of 2,5,6-tetrafluoropyridine
Applicaion
We consider that an organo-aluminium intermediate is Moreover, various 4-substituted tetrachloropyritlines involved in the reduction of (I) containing an AlH, group of which examples have been cited12-rather than a lithio derivative of polychloropyridine which requires low temperature for its format. Thus on the basis of the described experimental evidence the reduction of pentachloropyridine (I) can be rationalized as set out (picture 1) : tram--addition across the 3-4 bond which no doubt possesses a degree of olefinic character gives the aluminium-containing intermediate (IX) which at room temperature for 16 hr. suffers reduction of either the ‘allylic’ 4-, or 3-chlorine atom to give (X) or (XI) with inversion. Further reduction to give (XII) can also occur. Addition of H, O, D, O, or CO, causes (4-X-C5Cl4, N; X = NHEt, C5H10N, OMe) were attacked by LiAlH, at the 3- or under more drastic conditions, the 3- and 4-positions. These results are equally well accommodated by analogy with the reaction scheme and will be reported in detail with others still under investigation. We also observed exclusive formation of 2,5,6-tetrachloropyridine by LiAlH4 reduction of pentachloropyridine N-oxide presumably by 1, 2-addition.
Reference
1 R. E. Banks, J. E. Burgess, W. M. Cheng, and R. N. Haszeldine, J. Chem. SOG., 1965, 575.
2 J. D. Cook and B. J. Wakefield, J. Organometallic Chern., 1968, 13, 15.
3 K. J. Saunders, Dip. Tech. Thesis, Salford, 1967.
4 W. T. Flowers, R. N. Haszeldine, and S. A. Majid, Tetvahedron Letters, 1967, 2503.
5 S. M. Roberts, F. Roy, and H. Suschitzky, Meeting of the Heterocyclic Group of the Chem. SOC., London, January 1969.
6 D. J. Burton and J. Mettille, Inorg. Nuclear Chenz. Letters, 1968, 4, 9.
7 W. J. Sell, J. Chem. SOG., 1911, 99, 1679. D. Y. Curtin, J. A. Kampmeier, and B. R. O’Connor, J. Amer. Chem., 1965, 87, 863.
8 V. P. Danil'chuk and A. M. Sipyagin, in: Chemistry and Technology of Pyridine-Containing Pesticides. Collection of Papers [in Russian], No. 2, Chernogolovka (1988), p. 63.
Lastest Price from 2,5,6-tetrachloropyridine manufacturers

US $10.00/kg2025-04-21
- CAS:
- 54718-39-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00-0.00/kg2025-04-04
- CAS:
- 54718-39-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton