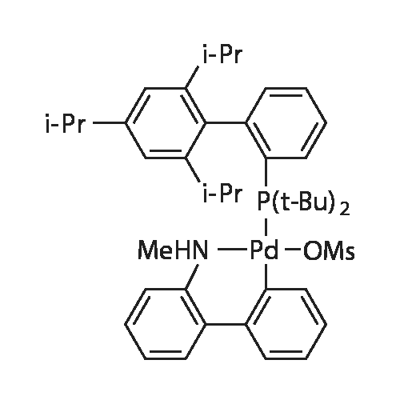
The introduction of t-BuXphos Palladacycle Gen. 4
General description
The t-BuXphos Palladacycle Gen. 4, with the CAS No: 1599466-89-3, is also known as Methanesulfonato(2-di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl)(2'-methylamino-1,1'-biphenyl-2-yl)palladium(II) dichloromethane adduct. This chemical’s molecular formula is C44H62Cl2NO3PPdS and molecular weight is 893.33. Its appearance is off-white to gray powder. It is a kind of palladium oxidative addition complexes and used to be a metal complex catalyst to catalyze C?C and C?N Cross-Couplings. Its structure is as follows:
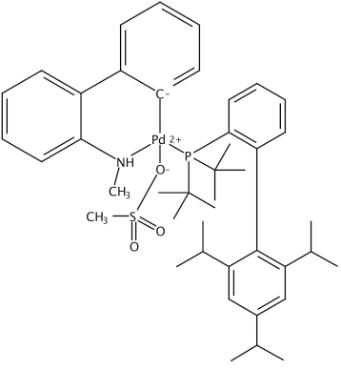
Figure 1 Structure of t-BuXphos Palladacycle Gen. 4.
Chemical synthesis of t-BuXphos Palladacycle Gen. 4
The t-BuXphos Palladacycle Gen. 4 can be synthesized by one step according to the previous work [1], and the synthesis route is shown in Figure 2. A 24 mL screw-top test tube equipped with a stir bar was charged with dimeric palladacycles (384 mg, 0.50 mmol, 0.50 equiv) XPhos (476 mg, 1.00 mmol, 1.00 equiv). Dichloromethane (5 mL) was added, and the reaction mixture was stirred at room temperature for 1 h. The solvent was removed with the aid of rotary evaporation. Pentane (25 mL) was added to the residue to precipitate the precatalyst, which was then isolated via vacuum filtration and dried under vacuum overnight. t-BuXphos Palladacycle Gen. 4. Light yellow solid. Yield: 720 mg, 89%. 1H NMR (500 MHz, CD3OD): δ 8.15 (t, J = 6.8 Hz, 1H), 7.93-6.80 (m, 13H), 3.44-3.32 (m, 1H), 3.14 (dt, J = 13.6, 6.8 Hz, 1H), 2.71 (m, 4H), 2.29-1.69 (m, 6H), 1.70-0.57 (m, 30H) ppm. 13C NMR (126 MHz, CD3CN): δ 160.4, 157.5, 153.9, 145.5, 145.3, 143.7, 142.0, 141.3, 139.4, 137.4, 137.4, 136.7, 135.5, 135.3, 135.0, 134.9, 132.04, 132.03, 129.8, 129.1, 128.93, 128.90, 128.8, 128.7, 128.5, 128.0, 127.9, 127.86, 127.0, 126.9, 126.4, 125.4, 124.8, 122.59, 122.58, 121.8, 43.84, 40.72, 40.71, 39.95, 39.73, 39.59, 39.47, 39.35, 35.05, 33.73, 32.21, 32.19, 32.15, 32.06, 30.82, 30.78, 26.18, 25.41, 24.28, 24.08, 24.04, 23.81, 23.26, 14.35 ppm (observed complexity due to C-P splitting). 31P NMR (121 MHz, DMSO-d6): δ 56.13 ppm. IR (neat, cm-1): 1247, 1143, 1031, 1018, 1001, 759, 747, 739, 729.
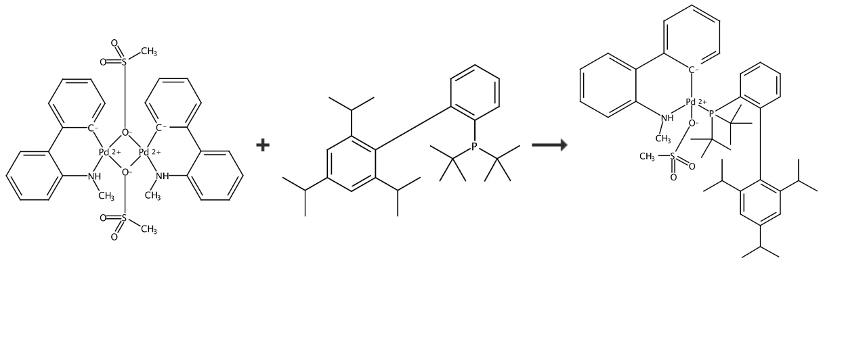
Figure 2 Chemical synthesis of t-BuXphos Palladacycle Gen. 4.
Application of t-BuXphos Palladacycle Gen. 4
As a metal complex catalyst, t-BuXphos Palladacycle Gen. 4 has been impactful for the functionalization of complex molecules. Densely functionalized biomolecules and pharmaceutical-like molecules often contain numerous heterocycles and nucleophilic functional groups that can act as ligands for transition metals [2]. When catalytic cross-coupling protocols with reaction conditions optimized using simple substrates are applied to complex molecules, they frequently fail to deliver product. Employing stoichiometric quantities of palladium OACs, like t-BuXphos Palladacycle Gen. 4, foregoes the need for multiple catalyst turnovers and allows for the reaction to start as close to the crucial bond-forming step as possible, therefore increasing the likelihood of reaction success. To this end, t-BuXphos Palladacycle Gen. 4 has been applied to late-stage diversification of pharmaceuticals [3], bioconjugation [4], and incorporation of radioisotopes [5]. The use of t-BuXphos Palladacycle Gen. 4 has allowed for the successful formation of challenging carbon?carbon and carbon-heteroatom bonds, a single OAC is not applicable to every desired bond formation [6]. The specific type of bonds that can be formed using a particular OAC are influenced by the ligand the OAC is generated with. No one ligand will facilitate every reductive elimination, as the unique steric and electronic environment that each ligand imparts on the metal center will favor one bond formation while disfavoring another. This ligand limitation is less of a concern when the OAC used is derived from a simple and abundant aryl halide, like t-BuXphos Palladacycle Gen. 4, as a large variety of uniquely ligated complexes can be readily generated.
References
[1]Bruno, NC, Niljianskul, N and Buchwald, SL. N-Substituted 2-Aminobiphenylpalladium Methanesulfonate Precatalysts and Their Use in C-C and C-N Cross-Couplings. Journal of organic chemistry, 2014, 79 (9): 4161-4166.
[2]Buitrago Santanilla, A.; Regalado, E. L.; Pereira, T.; Shevlin, M.; Bateman, K.; Campeau, L.-C.; Schneeweis, J.; Berritt, S.; Shi, Z.-C.; Nantermet, P.; Liu, Y.; Helmy, R.; Welch, C. J.; Vachal, P.; Davies, I. W.; Cernak, T.; Dreher, S. D. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 2015, 347, 49?53.
[3]Roque, J. B.; Kuroda, Y.; Jurczyk, J.; Xu, L.-P.; Ham, J. S.; Go?ttemann, L. T.; Roberts, C. A.; Adpressa, D.; Sauri?, J.; Joyce, L. A.; Musaev, D. G.; Yeung, C. S.; Sarpong, R. C-C Cleavage Approach to C-H Functionalization of Saturated Aza-Cycles. ACS Catal. 2020, 10, 2929?2941.
[4]Rojas, A. J.; Zhang, C.; Vinogradova, E. V.; Buchwald, N. H.; Reilly, J.; Pentelute, B. L.; Buchwald, S. L. Divergent unprotected peptide macrocyclisation by palladium-mediated cysteine arylation. Chem. Sci. 2017, 8, 4257?4263.
[5]Zhao, W.; Lee, H. G.; Buchwald, S. L.; Hooker, J. M. Direct 11CN-Labeling of Unprotected Peptides via Palladium-Mediated Sequential Cross-Coupling Reactions. J. Am. Chem. Soc. 2017, 139, 7152?7155.
[6]Ruiz-Castillo, P.; Buchwald, S. L. Applications of Palladium- Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564?12649.
See also
Lastest Price from t-BuXphos Palladacycle Gen. 4 manufacturers

US $2.00-5.00/kg2025-06-19
- CAS:
- 1599466-89-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg