The Biological activity of Arachidonic acid
Description
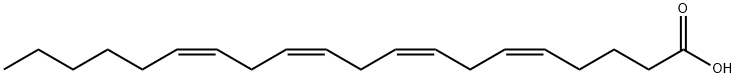
Arachidonic acid (ARA) is a 20-carbon chain fatty acid with four methylene-interrupted cis double bonds; the first, concerning the methyl end (omega, ω, or n), is located between carbon 6 and 7. Hence, ARA belongs to the omega-6 (n-6) polyunsaturated fatty acids (PUFA), is designated as 20:4ω-6, with a biochemical nomenclature of all-cis-5,8,11,14-eicosatetraenoic acid, and usually assumes a hairpin configuration.
Source
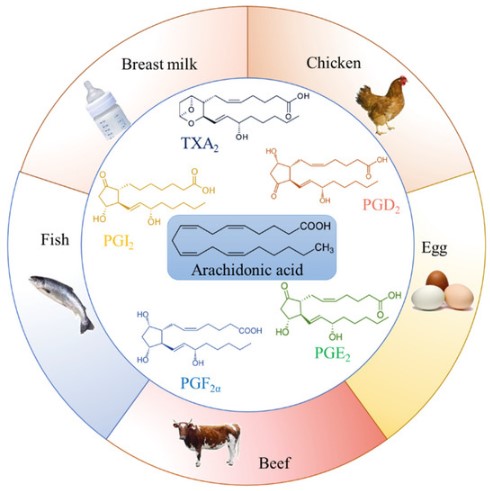
ARA is obtained from poultry, animal organs, and meat, fish, seafood, and eggs. It is incorporated in phospholipids in the cells' cytosol. It is adjacent to the endoplasmic reticulum membrane studded with the proteins necessary for phospholipid synthesis and their allocation to the diverse biological membranes. Another important ARA source for herbivores and vegetarians is linoleic acid, an omega-6, 18-carbonated PUFA that contains only two cis-double bonds (18:2ω-6)[1].
Biological activity
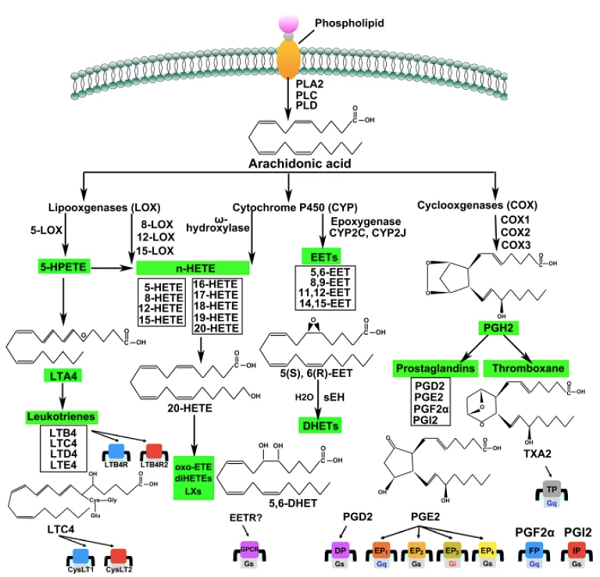
ARA, a polyunsaturated fatty acid in the phospholipids of cell membranes, is an essential inflammatory mediator involved in many molecular and cellular functions under physiological and pathological conditions. Although ARA signaling regulates the activities of many TRP channels, including TRPV1 and TRPV4, different ARA metabolic pathways are involved[2]. For instance, activation of bradykinin B2 receptors produces 12-lipoxygenase metabolites such as 12-HPETE to activate TRPV1 and increase the excitability of sensory nerve endings. On the other hand, the endocannabinoid anandamide and ARA use the cytochrome P450 epoxygenase-dependent formation of epoxyeicosatrienoic acids such as 5′, 6′-epoxyeicosatrienoic acid (EET) and 11′, 12′-EET to activate TRPV4.
Metabolic process
Arachidonic acid and its metabolites (prostaglandins and leukotrienes) are now considered intracellular messengers. Arachidonic acid is an essential fatty acid and a precursor in the biosynthesis of prostaglandins, thromboxanes, and leukotrienes Bosetti[3-4]. Stimulating specific cell-surface receptors activates phospholipase A2, releasing arachidonic acid from the cell membrane. Cyclooxygenases rapidly convert The arachidonic acid into active metabolites to produce prostaglandins, prostacyclin, and thromboxanes, and lipoxygenase to produce leukotrienes Page et al.
References
[1] Hatem Tallima , Rashika El Ridi. “Arachidonic acid: Physiological roles and potential health benefits – A review.” Journal of Advanced Research 11 (2018): Pages 33-41.
[2] Jialie Luo, Hongzhen Hu. “Thermally activated TRPV3 channels.” Current topics in membranes (2014): 325–64.
[3] Chunjie Xu. “FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer.” Nature Communications 14 1 (2023): 2042.
[4] Kevin B Hadley. “The Essentiality of Arachidonic Acid in Infant Development.” Nutrients 8 4 (2016): 216.
You may like
Related articles And Qustion
See also
Lastest Price from Arachidonic acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 506-32-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 506-32-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt